Mapping the Morphological Landscape of Oligomeric Di-block Peptide-Polymer Amphiphiles
- PMID: 35037351
- PMCID: PMC8957712
- DOI: 10.1002/anie.202115547
Mapping the Morphological Landscape of Oligomeric Di-block Peptide-Polymer Amphiphiles
Abstract
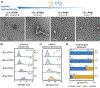
Peptide-polymer amphiphiles (PPAs) are tunable hybrid materials that achieve complex assembly landscapes by combining the sequence-dependent properties of peptides with the structural diversity of polymers. Despite their promise as biomimetic materials, determining how polymer and peptide properties simultaneously affect PPA self-assembly remains challenging. We herein present a systematic study of PPA structure-assembly relationships. PPAs containing oligo(ethyl acrylate) and random-coil peptides were used to determine the role of oligomer molecular weight, dispersity, peptide length, and charge density on self-assembly. We observed that PPAs predominantly formed spheres rather than anisotropic particles. Oligomer molecular weight and peptide hydrophilicity dictated morphology, while dispersity and peptide charge affected particle size. These key benchmarks will facilitate the rational design of PPAs that expand the scope of biomimetic functionality within assembled soft materials.
Keywords: Biomimetic; Electron Microscopy; Peptide-Polymer Amphiphile; Self-Assembly; Supramolecular Chemistry.
© 2022 Wiley-VCH GmbH.
Figures





References
-
- Barbee MH, Wright ZM, Allen BP, Taylor HF, Patteson EF, Knight AS, Macromolecules 2021, 54, 3585–3612
-
- Mai Y, Eisenberg A, Chem. Soc. Rev 2012, 41, 5969–5985. - PubMed
-
- Krieg E, Bastings MMC, Besenius P, Rybtchinski B, Chem. Rev 2016, 116, 2414–2477. - PubMed
-
- Chen C, Wylie RALL, Klinger D, Connal LA, Chem. Mater 2017, 29, 1918–1945.
-
- Nicolai T, Colombani O, Chassenieux C, Soft Matter 2010, 6, 3111–3118.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

