Epidemiological trends of HIV/HCV coinfection in Spain, 2015-2019
- PMID: 35037379
- PMCID: PMC9543728
- DOI: 10.1111/hiv.13229
Epidemiological trends of HIV/HCV coinfection in Spain, 2015-2019
Abstract
Objectives: We assessed the prevalence of anti-hepatitis C virus (HCV) antibodies and active HCV infection (HCV-RNA-positive) in people living with HIV (PLWH) in Spain in 2019 and compared the results with those of four similar studies performed during 2015-2018.
Methods: The study was performed in 41 centres. Sample size was estimated for an accuracy of 1%. Patients were selected by random sampling with proportional allocation.
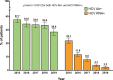
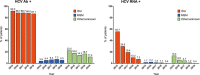
Results: The reference population comprised 41 973 PLWH, and the sample size was 1325. HCV serostatus was known in 1316 PLWH (99.3%), of whom 376 (28.6%) were HCV antibody (Ab)-positive (78.7% were prior injection drug users); 29 were HCV-RNA-positive (2.2%). Of the 29 HCV-RNA-positive PLWH, infection was chronic in 24, it was acute/recent in one, and it was of unknown duration in four. Cirrhosis was present in 71 (5.4%) PLWH overall, three (10.3%) HCV-RNA-positive patients and 68 (23.4%) of those who cleared HCV after anti-HCV therapy (p = 0.04). The prevalence of anti-HCV antibodies decreased steadily from 37.7% in 2015 to 28.6% in 2019 (p < 0.001); the prevalence of active HCV infection decreased from 22.1% in 2015 to 2.2% in 2019 (p < 0.001). Uptake of anti-HCV treatment increased from 53.9% in 2015 to 95.0% in 2019 (p < 0.001).
Conclusions: In Spain, the prevalence of active HCV infection among PLWH at the end of 2019 was 2.2%, i.e. 90.0% lower than in 2015. Increased exposure to DAAs was probably the main reason for this sharp reduction. Despite the high coverage of treatment with direct-acting antiviral agents, HCV-related cirrhosis remains significant in this population.
Keywords: HIV infection/*epidemiology; coinfection/*epidemiology; hepatitis C/drug therapy/*epidemiology.
© 2022 The Authors. HIV Medicine published by John Wiley & Sons Ltd on behalf of British HIV Association.
Conflict of interest statement
JB reports honoraria for advice or public speaking from AbbVie, Gilead, MSD, Janssen and ViiV Healthcare; and grants from AbbVie, Gilead, MSD and ViiV Healthcare. AR‐R reports honoraria for advice or public speaking from AbbVie, Gilead, MSD, Janssen and ViiV Healthcare, and grants from AbbVie, Gilead, MSD, Janssen and ViiV Healthcare. IJ reports honoraria for advice or public speaking from Gilead and ViiV Healthcare. JGG reports honoraria for advice or public speaking from AbbVie, Gilead, MSD, Janssen and ViiV Healthcare, and grants from Gilead, MSD and ViiV Healthcare. The rest of the authors have nothing to disclose.
Figures
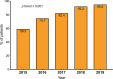
References
-
- Sulkowski MS, Mast EE, Seeff LB, Thomas DL. Hepatitis C virus infection as an opportunistic disease in persons infected with human immunodeficiency virus. Clin Infect Dis. 2000;30(Suppl 1):S77‐S84. - PubMed
-
- Boesecke C, Rockstroh JK. Acute hepatitis C in patients with HIV. Semin Liver Dis. 2012;32(2):130‐137. - PubMed
-
- Aisyah DN, Shallcross L, Hully AJ, O'Brien A, Hayward A. Assessing hepatitis C spontaneous clearance and understanding associated factors‐A systematic review and meta‐analysis. J Viral Hepat. 2018;25(6):680‐698. - PubMed
-
- Benhamou Y, Bochet M, Di Martino V, et al. Liver fibrosis progression in human immunodeficiency virus and hepatitis C virus coinfected patients. The Multivirc group. Hepatology. 1999;30(4):1054‐1058. - PubMed
-
- Graham CS, Baden LR, Yu E, et al. Influence of human immunodeficiency virus infection on the course of hepatitis C virus infection: a meta‐analysis. Clin Infect Dis. 2001;33(4):562‐569. - PubMed

