Faster Crystallization during Coral Skeleton Formation Correlates with Resilience to Ocean Acidification
- PMID: 35037457
- PMCID: PMC8796227
- DOI: 10.1021/jacs.1c11434
Faster Crystallization during Coral Skeleton Formation Correlates with Resilience to Ocean Acidification
Abstract
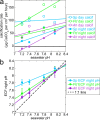
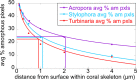
The mature skeletons of hard corals, termed stony or scleractinian corals, are made of aragonite (CaCO3). During their formation, particles attaching to the skeleton's growing surface are calcium carbonate, transiently amorphous. Here we show that amorphous particles are observed frequently and reproducibly just outside the skeleton, where a calicoblastic cell layer envelops and deposits the forming skeleton. The observation of particles in these locations, therefore, is consistent with nucleation and growth of particles in intracellular vesicles. The observed extraskeletal particles range in size between 0.2 and 1.0 μm and contain more of the amorphous precursor phases than the skeleton surface or bulk, where they gradually crystallize to aragonite. This observation was repeated in three diverse genera of corals, Acropora sp., Stylophora pistillata─differently sensitive to ocean acidification (OA)─and Turbinaria peltata, demonstrating that intracellular particles are a major source of material during the additive manufacturing of coral skeletons. Thus, particles are formed away from seawater, in a presumed intracellular calcifying fluid (ICF) in closed vesicles and not, as previously assumed, in the extracellular calcifying fluid (ECF), which, unlike ICF, is partly open to seawater. After particle attachment, the growing skeleton surface remains exposed to ECF, and, remarkably, its crystallization rate varies significantly across genera. The skeleton surface layers containing amorphous pixels vary in thickness across genera: ∼2.1 μm in Acropora, 1.1 μm in Stylophora, and 0.9 μm in Turbinaria. Thus, the slow-crystallizing Acropora skeleton surface remains amorphous and soluble longer, including overnight, when the pH in the ECF drops. Increased skeleton surface solubility is consistent with Acropora's vulnerability to OA, whereas the Stylophora skeleton surface layer crystallizes faster, consistent with Stylophora's resilience to OA. Turbinaria, whose response to OA has not yet been tested, is expected to be even more resilient than Stylophora, based on the present data.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Sun C.-Y.; Stifler C. A.; Chopdekar R. V.; Schmidt C. A.; Parida G.; Schoeppler V.; Fordyce B. I.; Brau J. H.; Mass T.; Tambutté S.; Gilbert P. U. P. A. From particle attachment to space-filling coral skeletons. Proc. Natl. Acad. Sci. 2020, 117 (48), 30159–30170. 10.1073/pnas.2012025117. - DOI - PMC - PubMed
-
- Tambutté E.; Allemand D.; Zoccola D.; Meibom A.; Lotto S.; Caminiti N.; Tambutté S. Observations of the tissue-skeleton interface in the scleractinian coral Stylophora pistillata. Coral Reefs 2007, 26 (3), 517–529. 10.1007/s00338-007-0263-5. - DOI

