Recirculating hyperthermic intravesical chemotherapy with mitomycin C (HIVEC) versus BCG in high-risk non-muscle-invasive bladder cancer: results of the HIVEC-HR randomized clinical trial
- PMID: 35037963
- PMCID: PMC8994727
- DOI: 10.1007/s00345-022-03928-1
Recirculating hyperthermic intravesical chemotherapy with mitomycin C (HIVEC) versus BCG in high-risk non-muscle-invasive bladder cancer: results of the HIVEC-HR randomized clinical trial
Abstract
Purpose: The purpose of the study was to compare the outcomes of high-risk non-muscle-invasive bladder cancer (HR-NMIBC) patients treated with BCG vs recirculating hyperthermic intravesical chemotherapy (HIVEC) with mitomycin C (MMC).
Methods: A pilot phase II randomized clinical trial was conducted including HR-NMIBC patients, excluding carcinoma in situ. Patients were randomized 1:1 to receive intravesical BCG for 1 year (once weekly for 6 weeks plus subsequent maintenance) or HIVEC with 40 mg MMC, administered using the Combat BRS system (once weekly instillations were given for 6 weeks, followed by once monthly instillation for 6 months). Total recirculating dwell time for HIVEC was 60 min at a target temperature of 43° ± 0.5 °C. Primary endpoint was recurrence-free survival. Secondary endpoints were time to recurrence, progression-free survival, cancer-specific survival, and overall survival at 24 months. Adverse events were routinely assessed.
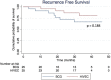
Results: Fifty patients were enrolled. Mean age was 73.5 years. Median follow-up was 33.7 months. Recurrence-free survival at 24 months was 86.5% for HIVEC and 71.8% for BCG (p = 0.184) in the intention-to-treat analysis and 95.0% for HIVEC and 75.1% for BCG (p = 0.064) in the per protocol analysis. Time to recurrence was 21.5 and 16.1 months for HIVEC and BCG, respectively. Progression-free survival for HIVEC vs BCG was 95.7% vs 71.8% (p = 0.043) in the intention-to-treat analysis and 100% vs 75.1% (p = 0.018) in the per protocol analysis, respectively. Cancer-specific survival at 24 months was 100% for both groups and overall survival was 91.5% for HIVEC vs 81.8% for BCG.
Conclusion: HIVEC provides comparable safety and efficacy to BCG and is a reasonable alternative during BCG shortages.
Trial registration: EudraCT 2016-001186-85. Date of registration: 17 March 2016.
Keywords: Bacillus Calmette–Guérin; Bladder cancer; Hyperthermia; Progression; Recurrence.
© 2022. The Author(s).
Conflict of interest statement
F. Guerrero-Ramos and B. A. Inman have received consultancy honoraria from Combat Medical Ltd.
Figures
Similar articles
-
Adjuvant intravesical treatment in patients with intermediate and high-risk non-muscle-invasive bladder cancer with BCG versus MMC applied with COMBAT or EMDA. Results of a prospective study.J Cancer Res Clin Oncol. 2023 Aug;149(10):7453-7459. doi: 10.1007/s00432-023-04688-0. Epub 2023 Mar 23. J Cancer Res Clin Oncol. 2023. PMID: 36952006 Free PMC article.
-
Comparison of hyperthermic intravesical chemotherapy and Bacillus Calmette-Guerin therapy in high-risk non-muscle invasive bladder cancer: a matched-pair analysis.Int Urol Nephrol. 2024 Mar;56(3):957-963. doi: 10.1007/s11255-023-03849-x. Epub 2023 Oct 25. Int Urol Nephrol. 2024. PMID: 37880493
-
Clinical trial of high dose hyperthermic intravesical mitomycin C for intermediate and high-risk non-muscle invasive bladder cancer during BCG shortage.Urol Oncol. 2021 Aug;39(8):498.e13-498.e20. doi: 10.1016/j.urolonc.2020.12.025. Epub 2021 Jan 21. Urol Oncol. 2021. PMID: 33485761
-
The Efficacy and Safety of Hyperthermia Intravesical Chemotherapy in the Treatment of Non-Muscle-Invasive Bladder Cancer: A Meta-Analysis.Urol Int. 2024;108(4):322-333. doi: 10.1159/000538373. Epub 2024 Mar 20. Urol Int. 2024. PMID: 38508149
-
Intravesical gemcitabine for non-muscle invasive bladder cancer.Cochrane Database Syst Rev. 2021 Jun 14;6(6):CD009294. doi: 10.1002/14651858.CD009294.pub3. Cochrane Database Syst Rev. 2021. PMID: 34125951 Free PMC article.
Cited by
-
Long-Term Outcomes of Intravesical Mitomycin C Administered via Electromotive Drug Administration or Conductive Chemo-Hyperthermia in Non-Muscle-Invasive Bladder Cancer.Cancers (Basel). 2025 Jan 28;17(3):453. doi: 10.3390/cancers17030453. Cancers (Basel). 2025. PMID: 39941820 Free PMC article.
-
Intravesical thermochemotherapy in the treatment of high-risk and very high-risk non-muscle-invasive urothelial bladder cancer: a single-arm study.Int Urol Nephrol. 2024 Jul;56(7):2243-2250. doi: 10.1007/s11255-023-03924-3. Epub 2024 Feb 8. Int Urol Nephrol. 2024. PMID: 38329573 Free PMC article.
-
Adjuvant intravesical treatment in patients with intermediate and high-risk non-muscle-invasive bladder cancer with BCG versus MMC applied with COMBAT or EMDA. Results of a prospective study.J Cancer Res Clin Oncol. 2023 Aug;149(10):7453-7459. doi: 10.1007/s00432-023-04688-0. Epub 2023 Mar 23. J Cancer Res Clin Oncol. 2023. PMID: 36952006 Free PMC article.
-
Is CIS a Contraindication to Hyperthermic Intravesical Chemotherapy (HIVEC) after BCG-Failure?Cancers (Basel). 2023 Feb 24;15(5):1455. doi: 10.3390/cancers15051455. Cancers (Basel). 2023. PMID: 36900247 Free PMC article.
-
Adjuvant Hyperthermic Intravesical Chemotherapy in Intermediate- and High-Risk Non-muscle Invasive Bladder Cancer.Cureus. 2023 Sep 21;15(9):e45672. doi: 10.7759/cureus.45672. eCollection 2023 Sep. Cureus. 2023. PMID: 37745737 Free PMC article.
References
-
- Cambier S, Sylvester RJ, Collette L, et al. EORTC Nomograms and risk groups for predicting recurrence, progression, and disease-specific and overall survival in non-muscle-invasive stage Ta-T1 urothelial bladder cancer patients treated with 1–3 years of maintenance bacillus Calmette-Guérin. Eur Urol. 2016;69(1):60–69. doi: 10.1016/j.eururo.2015.06.045. - DOI - PubMed
-
- Oddens J, Brausi M, Sylvester R, et al. Final results of an EORTC-GU cancers group randomized study of maintenance bacillus Calmette-Guérin in intermediate- and high-risk Ta, T1 papillary carcinoma of the urinary bladder: one-third dose versus full dose and 1 year versus 3 years of maintenance. Eur Urol. 2013;63(3):462–472. doi: 10.1016/j.eururo.2012.10.039. - DOI - PubMed
-
- Malmström PU, Sylvester RJ, Crawford DE, et al. An individual patient data meta-analysis of the long-term outcome of randomised studies comparing intravesical mitomycin C versus bacillus Calmette-Guérin for non-muscle-invasive bladder cancer. Eur Urol. 2009;56(2):247–256. doi: 10.1016/j.eururo.2009.04.038. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical