Algorithmic Approach to the Diagnosis of Organizing Pneumonia: A Correlation of Clinical, Radiologic, and Pathologic Features
- PMID: 35038455
- PMCID: PMC9899643
- DOI: 10.1016/j.chest.2021.12.659
Algorithmic Approach to the Diagnosis of Organizing Pneumonia: A Correlation of Clinical, Radiologic, and Pathologic Features
Abstract
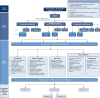
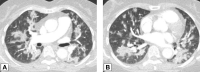
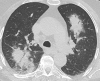
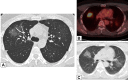
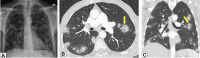
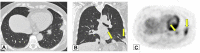
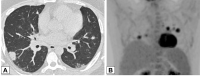
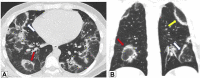
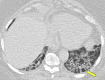
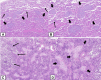
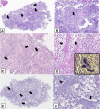
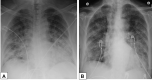
Organizing pneumonia (OP), characterized histopathologically by patchy filling of alveoli and bronchioles by loose plugs of connective tissue, may be seen in a variety of conditions. These include but are not limited to after an infection, drug reactions, radiation therapy, and collagen vascular diseases. When a specific cause is responsible for this entity, it is referred to as "secondary OP." When an extensive search fails to reveal a cause, it is referred to as "cryptogenic OP" (previously called "bronchiolitis obliterans with OP"), which is a clinical, radiologic, and pathologic entity classified as an interstitial lung disease. The clinical presentation of OP often mimics that of other disorders, such as infection and cancer, which can result in a delay in diagnosis and inappropriate management of the underlying disease. The radiographic presentation of OP is polymorphous but often has subpleural consolidations with air bronchograms or solitary or multiple nodules, which can wax and wane. Diagnosis of OP sometimes requires histopathologic confirmation and exclusion of other possible causes. Treatment usually requires a prolonged steroid course, and disease relapse is common. The aim of this article is to summarize the clinical, radiographic, and histologic presentations of this disease and to provide a practical diagnostic algorithmic approach incorporating clinical history and characteristic imaging patterns.
Keywords: acute fibrinous and organizing pneumonia; cryptogenic organizing pneumonia; focal organizing pneumonia; organizing pneumonia; secondary organizing pneumonia.
Copyright © 2022 American College of Chest Physicians. Published by Elsevier Inc. All rights reserved.
Figures
















References
-
- Cottin V., Cordier J.F. Cryptogenic organizing pneumonia. Semin Respir Crit Care Med. 2012;33(5):462–475. - PubMed
-
- Davison A.G., Heard B.E., McAllister W.A., Turner-Warwick M.E. Cryptogenic organizing pneumonitis. Q J Med. 1983;52(207):382–394. - PubMed
-
- Epler G.R., Colby T.V., McLoud T.C., Carrington C.B., Gaensler E.A. Bronchiolitis obliterans organizing pneumonia. N Engl J Med. 1985;312(3):152–158. - PubMed
-
- American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. Am J Respir Crit Care Med. 2002;165(2):277–304. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

