Randomized trial of three IVIg doses for treating chronic inflammatory demyelinating polyneuropathy
- PMID: 35038723
- PMCID: PMC9050528
- DOI: 10.1093/brain/awab422
Randomized trial of three IVIg doses for treating chronic inflammatory demyelinating polyneuropathy
Abstract
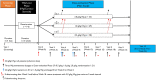
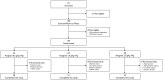
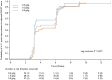
Intravenous immunoglobulin treatment for chronic inflammatory demyelinating polyneuropathy usually starts with a 2.0 g/kg induction dose followed by 1.0 g/kg maintenance doses every 3 weeks. No dose-ranging studies with intravenous immunoglobulin maintenance therapy have been published. The Progress in Chronic Inflammatory Demyelinating polyneuropathy (ProCID) study was a prospective, double-blind, randomized, parallel-group, multicentre, phase III study investigating the efficacy and safety of 10% liquid intravenous immunoglobulin (Panzyga®) in patients with active chronic inflammatory demyelinating polyneuropathy. Patients were randomized 1:2:1 to receive the standard intravenous immunoglobulin induction dose and then either 0.5, 1.0 or 2.0 g/kg maintenance doses every 3 weeks. The primary end point was the response rate in the 1.0 g/kg group, defined as an improvement ≥1 point in adjusted Inflammatory Neuropathy Cause and Treatment score at Week 6 versus baseline and maintained at Week 24. Secondary end points included dose response and safety. This trial was registered with EudraCT (Number 2015-005443-14) and clinicaltrials.gov (NCT02638207). Between August 2017 and September 2019, the study enrolled 142 patients. All 142 were included in the safety analyses. As no post-infusion data were available for three patients, 139 were included in the efficacy analyses, of whom 121 were previously on corticosteroids. The response rate was 80% (55/69 patients) [95% confidence interval (CI): 69-88%] in the 1.0 g/kg group, 65% (22/34; CI: 48-79%) in the 0.5 g/kg group, and 92% (33/36; CI: 78-97%) in the 2.0 g/kg group. While the proportion of responders was higher with higher maintenance doses, logistic regression analysis showed that the effect on response rate was driven by a significant difference between the 0.5 and 2.0 g/kg groups, whereas the response rates in the 0.5 and 2.0 g/kg groups did not differ significantly from the 1.0 g/kg group. Fifty-six per cent of all patients had an adjusted Inflammatory Neuropathy Cause and Treatment score improvement 3 weeks after the induction dose alone. Treatment-related adverse events were reported in 16 (45.7%), 32 (46.4%) and 20 (52.6%) patients in the 0.5, 1.0 and 2.0 g/kg dose groups, respectively. The most common adverse reaction was headache. There were no treatment-related deaths. Intravenous immunoglobulin (1.0 g/kg) was efficacious and well tolerated as maintenance treatment for patients with chronic inflammatory demyelinating polyneuropathy. Further studies of different maintenance doses of intravenous immunoglobulin in chronic inflammatory demyelinating polyneuropathy are warranted.
Keywords: Panzyga®; ProCID study; chronic inflammatory demyelinating polyneuropathy; intravenous immunoglobulin; randomized controlled trial.
© The Author(s) (2022). Published by Oxford University Press on behalf of the Guarantors of Brain.
Figures
References
-
- van den Bergh PYK, Hadden RDM, Bouche P, et al. ; Peripheral Nerve Society. European Federation of Neurological Societies/Peripheral Nerve Society guideline on management of chronic inflammatory demyelinating polyradiculoneuropathy: Report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society - first revision. Eur J Neurol. 2010;17(3):356–363. - PubMed
-
- van den Bergh PYK, van Doorn PA, Hadden RDM, et al. . European Academy of Neurology/Peripheral Nerve Society Guideline on diagnosis and treatment of chronic inflammatory demyelinating polyradiculoneuropathy: Report of a joint Task Force - second revision. J Peripher Nerv Syst. 2021;26(3):242–268. - PubMed
-
- Allen JA, Lewis RA. CIDP diagnostic pitfalls and perception of treatment benefit. Neurology. 2015;85(6):498–504. - PubMed
-
- Neligan A, Reilly MM, Lunn MP. CIDP: Mimics and chameleons. Pract Neurol. 2014;14(6):399–408. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials