Human Nasal Epithelial Cells Sustain Persistent SARS-CoV-2 Infection In Vitro, despite Eliciting a Prolonged Antiviral Response
- PMID: 35038898
- PMCID: PMC8764519
- DOI: 10.1128/mbio.03436-21
Human Nasal Epithelial Cells Sustain Persistent SARS-CoV-2 Infection In Vitro, despite Eliciting a Prolonged Antiviral Response
Abstract
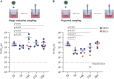
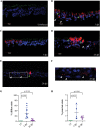
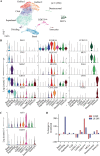
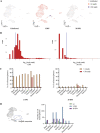
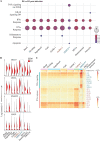
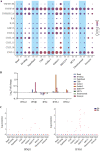
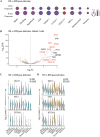
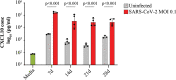
The dynamics of SARS-CoV-2 infection in COVID-19 patients are highly variable, with a subset of patients demonstrating prolonged virus shedding, which poses a significant challenge for disease management and transmission control. In this study, the long-term dynamics of SARS-CoV-2 infection were investigated using a human well-differentiated nasal epithelial cell (NEC) model of infection. NECs were observed to release SARS-CoV-2 virus onto the apical surface for up to 28 days postinfection (dpi), further corroborated by viral antigen staining. Single-cell transcriptome sequencing (sc-seq) was utilized to explore the host response from infected NECs after short-term (3-dpi) and long-term (28-dpi) infection. We identified a unique population of cells harboring high viral loads present at both 3 and 28 dpi, characterized by expression of cell stress-related genes DDIT3 and ATF3 and enriched for genes involved in tumor necrosis factor alpha (TNF-α) signaling and apoptosis. Remarkably, this sc-seq analysis revealed an antiviral gene signature within all NEC cell types even at 28 dpi. We demonstrate increased replication of basal cells, absence of widespread cell death within the epithelial monolayer, and the ability of SARS-CoV-2 to replicate despite a continuous interferon response as factors likely contributing to SARS-CoV-2 persistence. This study provides a model system for development of therapeutics aimed at improving viral clearance in immunocompromised patients and implies a crucial role for immune cells in mediating viral clearance from infected epithelia. IMPORTANCE Increasing medical attention has been drawn to the persistence of symptoms (long-COVID syndrome) or live virus shedding from subsets of COVID-19 patients weeks to months after the initial onset of symptoms. In vitro approaches to model viral or symptom persistence are needed to fully dissect the complex and likely varied mechanisms underlying these clinical observations. We show that in vitro differentiated human NECs are persistently infected with SARS-CoV-2 for up to 28 dpi. This viral replication occurred despite the presence of an antiviral gene signature across all NEC cell types even at 28 dpi. This indicates that epithelial cell intrinsic antiviral responses are insufficient for the clearance of SARS-CoV-2, implying an essential role for tissue-resident and infiltrating immune cells for eventual viral clearance from infected airway tissue in COVID-19 patients.
Keywords: COVID-19; SARS-CoV-2; nasal epithelial cells; single-cell sequencing; viral persistence.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Harvey WT, Carabelli AM, Jackson B, Gupta RK, Thomson EC, Harrison EM, Ludden C, Reeve R, Rambaut A, Consortium C-GU, Peacock SJ, Robertson DL, COVID-19 Genomics UK (COG-UK) Consortium . 2021. SARS-CoV-2 variants, spike mutations and immune escape. Nat Rev Microbiol 19:409–424. doi:10.1038/s41579-021-00573-0. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
