A Toxin-Conjugated Recombinant Protein Targeting gp120 and gp41 for Inactivating HIV-1 Virions and Killing Latency-Reversing Agent-Reactivated Latent Cells
- PMID: 35038908
- PMCID: PMC8764533
- DOI: 10.1128/mbio.03384-21
A Toxin-Conjugated Recombinant Protein Targeting gp120 and gp41 for Inactivating HIV-1 Virions and Killing Latency-Reversing Agent-Reactivated Latent Cells
Abstract
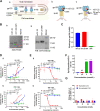
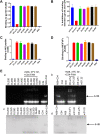
Application of the combination antiretroviral therapy (cART) has reduced AIDS to a manageable chronic infectious disease. However, HIV/AIDS cannot be cured because of the presence of latent reservoirs, thus calling for the development of antiretroviral drugs that can eliminate latency-reversing agent (LRA)-activated HIV-1 virions and latent cells. In this study, we conjugated a small-molecule toxin, DM1, to a gp120-binding protein, mD1.22, a mutated CD4 domain I, and found that mD1.22-DM1 could inactivate HIV-1 virions. However, it could not kill LRA-activated latent cells. We then designed and constructed a dual-targeting protein, DL35D, by linking mD1.22 and the single-chain variable fragment (scFv) of a gp41 NHR-specific antibody, D5, with a 35-mer linker. Subsequently, we conjugated DM1 to DL35D and found that DL35D-DM1 could inhibit HIV-1 infection, inactivate HIV-1 virions, kill HIV-1-infected cells and LRA-reactivated latent cells, suggesting that this toxin-conjugated dual-targeting recombinant protein is a promising candidate for further development as a novel antiviral drug with potential for HIV functional cure. IMPORTANCE Although HIV-1 replication was successfully controlled by antiretroviral drugs, cure strategy for HIV-1/AIDS is still lacking. The long-lived HIV reservoir is considered one of the major obstacles to an HIV/AIDS cure. CD4-PE40 was the first drug that designed to kill HIV-1 infected cells; however, lower efficiency and high immunogenicity have limited its further development. In this study, we designed several dual-targeting recombinant proteins DLDs by linking gp120-binding protein mD1.22 and gp41-binding antibody D5 scFv with different length of linkers. Among them, DL35D with 35-mer linker showed the best anti-HIV-1 activity. We further conjugated the DM1 toxin to DL35D to produce DL35D-DM1, which maintained DL35D's inhibitory and inactivation activity against cell-free HIV-1 strains. Most importantly, DL35D-DM1 could specifically kill HIV-1-infected cells and LRA-reactivated-latent infected cells, suggesting that it is a proper candidate for development as a novel antiviral drug for use in combination with an LRA for HIV functional cure.
Keywords: HIV-1; HIV-1 latent infection; shock and kill; toxin-conjugated recombinant protein; virus inactivator.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Synergistic Effect by Combining a gp120-Binding Protein and a gp41-Binding Antibody to Inactivate HIV-1 Virions and Inhibit HIV-1 Infection.Molecules. 2021 Mar 31;26(7):1964. doi: 10.3390/molecules26071964. Molecules. 2021. PMID: 33807292 Free PMC article.
-
Chimeric Antigen Receptor T Cells Guided by the Single-Chain Fv of a Broadly Neutralizing Antibody Specifically and Effectively Eradicate Virus Reactivated from Latency in CD4+ T Lymphocytes Isolated from HIV-1-Infected Individuals Receiving Suppressive Combined Antiretroviral Therapy.J Virol. 2016 Oct 14;90(21):9712-9724. doi: 10.1128/JVI.00852-16. Print 2016 Nov 1. J Virol. 2016. PMID: 27535056 Free PMC article.
-
TLR1/2 Agonist Enhances Reversal of HIV-1 Latency and Promotes NK Cell-Induced Suppression of HIV-1-Infected Autologous CD4+ T Cells.J Virol. 2021 Aug 10;95(17):e0081621. doi: 10.1128/JVI.00816-21. Epub 2021 Aug 10. J Virol. 2021. PMID: 34133900 Free PMC article.
-
Strategies to eradicate HIV from infected patients: elimination of latent provirus reservoirs.Cell Mol Life Sci. 2019 Sep;76(18):3583-3600. doi: 10.1007/s00018-019-03156-8. Epub 2019 May 25. Cell Mol Life Sci. 2019. PMID: 31129856 Free PMC article. Review.
-
Breaking the Silence: Regulation of HIV Transcription and Latency on the Road to a Cure.Viruses. 2023 Dec 15;15(12):2435. doi: 10.3390/v15122435. Viruses. 2023. PMID: 38140676 Free PMC article. Review.
Cited by
-
Alpaca-derived nanobody targeting the hydrophobic pocket of the HIV-1 gp41 NHR broadly neutralizes HIV-1 by blocking six-helix bundle formation.Curr Res Microb Sci. 2024 Jul 22;7:100263. doi: 10.1016/j.crmicr.2024.100263. eCollection 2024. Curr Res Microb Sci. 2024. PMID: 39176008 Free PMC article.
-
Peptide-Drug Conjugates: An Emerging Direction for the Next Generation of Peptide Therapeutics.J Med Chem. 2024 Feb 8;67(3):1641-1661. doi: 10.1021/acs.jmedchem.3c01835. Epub 2024 Jan 26. J Med Chem. 2024. PMID: 38277480 Free PMC article. Review.
-
Synaptic dysfunction is associated with alterations in the initiation of goal-directed behaviors: Implications for HIV-1-associated apathy.Exp Neurol. 2022 Nov;357:114174. doi: 10.1016/j.expneurol.2022.114174. Epub 2022 Jul 18. Exp Neurol. 2022. PMID: 35863502 Free PMC article.
-
Anti-HIV-1 Nanobody-IgG1 Constructs With Improved Neutralization Potency and the Ability to Mediate Fc Effector Functions.Front Immunol. 2022 May 16;13:893648. doi: 10.3389/fimmu.2022.893648. eCollection 2022. Front Immunol. 2022. PMID: 35651621 Free PMC article.
References
-
- Barré-Sinoussi F, Chermann JC, Rey F, Nugeyre MT, Chamaret S, Gruest J, Dauguet C, Axler-Blin C, Vézinet-Brun F, Rouzioux C, Rozenbaum W, Montagnier L. 1983. Isolation of a T-Lymphotropic Retrovirus From A Patient At Risk For Acquired Immune-Deficiency Syndrome (AIDS). Science 220:868–871. doi:10.1126/science.6189183. - DOI - PubMed
-
- Finzi D, Hermankova M, Pierson T, Carruth LM, Buck C, Chaisson RE, Quinn TC, Chadwick K, Margolick J, Brookmeyer R, Gallant J, Markowitz M, Ho DD, Richman DD, Siliciano RF. 1997. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science 278:1295–1300. doi:10.1126/science.278.5341.1295. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
