Pseudomonas aeruginosa C-Terminal Processing Protease CtpA Assembles into a Hexameric Structure That Requires Activation by a Spiral-Shaped Lipoprotein-Binding Partner
- PMID: 35038915
- PMCID: PMC8764530
- DOI: 10.1128/mbio.03680-21
Pseudomonas aeruginosa C-Terminal Processing Protease CtpA Assembles into a Hexameric Structure That Requires Activation by a Spiral-Shaped Lipoprotein-Binding Partner
Abstract
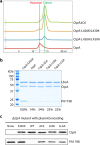
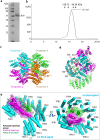
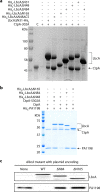
Pseudomonas aeruginosa CtpA is a carboxyl-terminal processing protease that partners with the outer membrane lipoprotein LbcA to degrade at least five cell wall-associated proteins, four of which are cell wall hydrolases. This activity plays an important role in supporting P. aeruginosa virulence in a mouse model of acute pneumonia. However, almost nothing is known about the molecular mechanisms underlying CtpA and LbcA function. Here, we used structural analysis to show that CtpA alone assembles into an inactive hexamer comprising a trimer of dimers, which limits its substrate access and prevents nonspecific degradation. The adaptor protein LbcA is a right-handed open spiral with 11 tetratricopeptide repeats, which might wrap around a substrate to deliver it to CtpA for degradation. By structure-guided mutagenesis and functional assays, we also showed that the interfaces of the CtpA trimer of dimers and an N-terminal helix of LbcA are important for LbcA-mediated substrate degradation by CtpA both in vitro and in vivo. This work improves our understanding of the molecular mechanism of the LbcA-CtpA proteolytic system and reveals some striking differences from the arrangements found in some other bacterial CTPs. IMPORTANCE Carboxyl-terminal processing proteases (CTPs) are found in all three domains of life. In bacteria, some CTPs have been associated with virulence, raising the possibility that they could be therapeutic targets. However, relatively little is known about their molecular mechanisms of action. In Pseudomonas aeruginosa, CtpA supports virulence by working in complex with the outer membrane lipoprotein LbcA to degrade cell wall hydrolases. Here, we report structure-function analyses of CtpA and LbcA, which reveals that CtpA assembles into an inactive hexamer comprising a trimer of dimers. LbcA is monomeric, with the first N-terminal helix important for binding to and activating CtpA, followed by a spiral structure composed of 11 tetratricopeptide repeats, which could wrap around a substrate for delivery to CtpA. This work reveals a unique mutimeric arrangement for a CTP and insight into how the important LbcA-CtpA proteolytic system functions.
Keywords: C-terminal processing protease; Pseudomonas aeruginosa; cell wall; cryo-EM; structural biology.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
