Intracranial efficacy of alectinib in ALK-positive NSCLC patients with CNS metastases-a multicenter retrospective study
- PMID: 35039026
- PMCID: PMC8764827
- DOI: 10.1186/s12916-021-02207-x
Intracranial efficacy of alectinib in ALK-positive NSCLC patients with CNS metastases-a multicenter retrospective study
Abstract
Background: Central nervous system (CNS) metastases in patients with ALK-positive non-small cell lung cancer (NSCLC) are a cause of substantial morbidity and mortality. Although alectinib had demonstrated promising intracranial efficacy in several clinical trials, data were limited on its CNS activity in real-world settings.
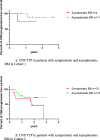
Methods: In this retrospective study, ALK-positive NSCLC patients with brain metastases (BM) or leptomeningeal metastases (LM) from six hospitals in China were divided into three cohorts based on the treatment history before the administration of alectinib. ALK-TKI-naive patients were enrolled in cohort 1, cohort 2 included patients who experienced intracranial progression with or without extracranial progression after treatment with crizotinib, and cohort 3 included patients who developed progression only in CNS following treatment with other second-generation ALK-TKIs. The definition and evaluation of intracranial and extracranial lesions were based on Response Evaluation Criteria in Solid Tumors version 1.1.
Results: Sixty-five patients were eligible and included in our study (cohort 1: 20, cohort 2: 32, cohort 3: 13). For the overall population and patients with uncontrolled CNS metastases, similar intracranial response in CNS target lesions was observed: cohort 1: 81.8% and 80%; cohort 2: 76.5% and 86.7%; cohort 3: 42.8% and 33.3%. For patients in these three cohorts, 75% (6/8), 78.6% (11/14), and 83.3% (5/6) were reported to have significant improvement in CNS-related symptoms respectively. The number of patients who were in need of mannitol or corticosteroids decreased remarkably after the treatment of alectinib (p < 0.001), and there was also a steep fall-over in the number of patients with ECOG ≥2 points before and after the administration of alectinib (p = 0.003). All patients (8/8) diagnosed with LM ± BM experienced substantial alleviation in CNS-related symptoms. In cohort 1 and cohort 2, no significant difference in CNS-time to progression was found between patients with symptomatic or asymptomatic BM when treated with alectinib alone.
Conclusions: Our study substantiated the potent CNS activity of alectinib in real-world settings. Patients with symptomatic and asymptomatic BM could benefit from alectinib comparatively, which indicated that alectinib alone might defer the timing of local treatment. However, our results should be treated cautiously owing to limited sample size.
Keywords: ALK-positive non-small cell lung cancer; Alectinib; Brain metastases; Central nervous system metastases; Leptomeningeal metastases.
© 2021. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
-
- Duruisseaux M, Besse B, Cadranel J, Perol M, Mennecier B, Bigay-Game L, Descourt R, Dansin E, Audigier-Valette C, Moreau L, et al. Overall survival with crizotinib and next-generation ALK inhibitors in ALK-positive non-small-cell lung cancer (IFCT-1302 CLINALK): a French nationwide cohort retrospective study. Oncotarget. 2017;8(13):21903–21917. doi: 10.18632/oncotarget.15746. - DOI - PMC - PubMed
-
- Ito K, Yamanaka T, Hayashi H, Hattori Y, Nishino K, Kobayashi H, Oya Y, Yokoyama T, Seto T, Azuma K, Fukui T, Kozuki T, Nakamura A, Tanaka K, Hirano K, Yokoi T, Daga H, Sakata S, Fujimoto D, Mori M, Maeno K, Aoki T, Tamura A, Miura S, Watanabe S, Akamatsu H, Hataji O, Suzuki K, Hontsu S, Azuma K, Bessho A, Kubo A, Okuno M, Nakagawa K, Yamamoto N. Sequential therapy of crizotinib followed by alectinib for non-small cell lung cancer harbouring anaplastic lymphoma kinase rearrangement (WJOG9516L): a multicenter retrospective cohort study. Eur J Cancer. 2021;145:183–193. doi: 10.1016/j.ejca.2020.12.026. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

