Potential for the Development of a New Generation of Aminoglycoside Antibiotics
- PMID: 35039693
- PMCID: PMC8754558
- DOI: 10.1007/s11094-021-02510-0
Potential for the Development of a New Generation of Aminoglycoside Antibiotics
Abstract
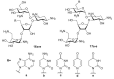
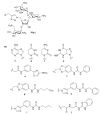
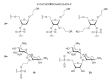
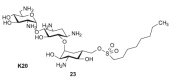
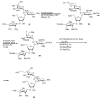
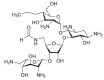
The present review summarizes recent publications devoted to aminoglycosides that study the main types of resistance to antibiotics of this class and the main directions of chemical modification aimed at overcoming the resistance or changing the spectrum of biological activity. Conjugates of aminoglycosides with various pharmacophores including amino acids, peptides, peptide nucleic acids, nucleic bases, and several other biologically active molecules and modifications resulting in other types of biological activity of this class of antibiotics are described. It is concluded that a promising research direction aimed at increasing the activity of antibiotics against resistant strains is the search for selective inhibitors of aminoglycoside-modifying enzymes. This would allow renewal of the use of antibiotics already meeting widespread resistance and would increase the potential of a new generation of antibiotics.
Keywords: aminoglycoside antibiotics; chemical modification; drug resistance; mechanism of action; structure–activity relationship.
© Springer Science+Business Media, LLC, part of Springer Nature 2021.
Figures



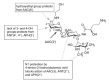
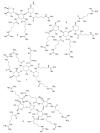

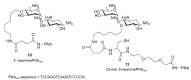

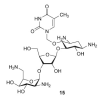

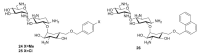
References
LinkOut - more resources
Full Text Sources
