Hyperbaric oxygen treatment improves pancreatic β‑cell function and hepatic gluconeogenesis in STZ‑induced type‑2 diabetes mellitus model mice
- PMID: 35039874
- PMCID: PMC8809048
- DOI: 10.3892/mmr.2022.12606
Hyperbaric oxygen treatment improves pancreatic β‑cell function and hepatic gluconeogenesis in STZ‑induced type‑2 diabetes mellitus model mice
Abstract
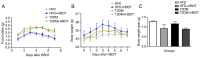
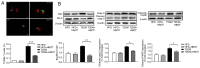
Type‑2 diabetes mellitus (T2DM) causes several complications that affect the quality of life and life span of patients. Hyperbaric oxygen therapy (HBOT) has been used to successfully treat several diseases, including carbon monoxide poisoning, ischemia, infections and diabetic foot ulcer, and increases insulin sensitivity in T2DM. The present study aimed to determine the effect of HBOT on β‑cell function and hepatic gluconeogenesis in streptozotocin (STZ)‑induced type‑2 diabetic mice. To establish a T2DM model, 7‑week‑old male C57BL/6J mice were fed a high‑fat diet (HFD) and injected once daily with low‑dose STZ for 3 days after 1‑week HFD feeding. At the 14th week, HFD+HBOT and T2DM+HBOT groups received 1‑h HBOT (2 ATA; 100% pure O2) daily from 5:00 to 6:00 p.m. for 7 days. The HFD and T2DM groups were maintained under normobaric oxygen conditions and used as controls. During HBOT, the 12‑h nocturnal food intake and body weight were measured daily. Moreover, blood glucose was measured by using a tail vein prick and a glucometer. After the final HBO treatment, all mice were sacrificed to conduct molecular biology experiments. Fasting insulin levels of blood samples of sacrificed mice were measured by an ultrasensitive ELISA kit. Pancreas and liver tissues were stained with hematoxylin and eosin, while immunohistochemistry was performed to determine the effects of HBOT on insulin resistance. TUNEL was used to determine the effects of HBOT on β‑cell apoptosis, and immunoblotting was conducted to determine the β‑cell apoptosis pathway. HBOT notably reduced fasting blood glucose and improved insulin sensitivity in T2DM mice. After HBOT, β‑cell area and β‑cell mass in T2DM mice were significantly increased. HBOT significantly decreased the β‑cell apoptotic rate in T2DM mice via the pancreatic Bcl‑2/caspase‑3/poly(ADP‑ribose) polymerase (PARP) apoptosis pathway. Moreover, HBOT improved the morphology of the liver tissue and increased hepatic glycogen storage in T2DM mice. These findings suggested that HBOT ameliorated the insulin sensitivity of T2DM mice by decreasing the β‑cell apoptotic rate via the pancreatic Bcl‑2/caspase‑3/PARP apoptosis pathway.
Keywords: Bcl-2/caspase-3/poly(ADP-ribose) polymerase pathway; hyperbaric oxygen; type-2 diabetes mellitus; β‑cell apoptosis.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

