Soluble neprilysin and survival in critically ill patients
- PMID: 35040286
- PMCID: PMC8934932
- DOI: 10.1002/ehf2.13787
Soluble neprilysin and survival in critically ill patients
Abstract
Background: Critically ill patients admitted to an intensive care unit (ICU) exhibit a high mortality rate irrespective of the initial cause of hospitalization. Neprilysin, a neutral endopeptidase degrading an array of vasoactive peptides became a drug target within the treatment of heart failure with reduced ejection fraction. The aim of this study was to analyse whether circulating levels of neprilysin at ICU admission are associated with 30 day mortality.
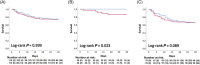
Methods and results: In this single-centre prospective observational study, 222 consecutive patients admitted to a tertiary ICU at a university hospital were included. Blood was drawn at admission and soluble neprilysin levels were measured using ELISA. In the total cohort, soluble neprilysin levels did not differ according to survival status after 30 days as well as type of admission. However, in patients after surgery or heart valve intervention, 30 day survivors exhibited significantly lower circulating neprilysin levels as compared to those who died within 30 days (660.2, IQR: 156.4-2512.5 pg/mL vs. 6532.6, IQR: 1840.1-10 000.0 pg/mL; P = 0.02). Soluble neprilysin predicted mortality independently from age, gender, and commonly used scores of risk-prediction (EuroSCORE II, STS-score, and SAPS II score). Additionally, soluble neprilysin was markedly elevated in patients with sepsis and septic shock (P < 0.05).
Conclusion: At the time of ICU admission, circulating levels of neprilysin independently predicted 30 day mortality in patients following cardiac surgery or heart valve intervention, but not in critically ill medical patients. Furthermore, patients suffering from sepsis and septic shock displayed significantly increased circulating neprilysin levels.
Keywords: 30 day mortality; Critical care; ICU; Neprilysin; Soluble neprilysin.
© 2022 The Authors. ESC Heart Failure published by John Wiley & Sons Ltd on behalf of European Society of Cardiology.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
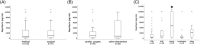

References
-
- Moss S, Subramanian V, Acharya KR. Crystal structure of peptide‐bound neprilysin reveals key binding interactions. FEBS Lett 2020; 594: 327–336. - PubMed
-
- Polna I, Aleksandrowicz J. Effect of adsorbents on IgM and IgG measles antibodies. Acta Virol 1975. Nov; 19: 449–456. - PubMed
-
- Ronco P, Pollard H, Galceran M, Delauche M, Schwartz JC, Verroust P. Distribution of enkephalinase (membrane metalloendopeptidase, E.C. 3.4.24.11) in rat organs. Detection using a monoclonal antibody. Lab Invest 1988; 58: 210–217. - PubMed
-
- Spillantini MG, Sicuteri F, Salmon S, Malfroy B. Characterization of endopeptidase 3.4.24.11 (“enkephalinase”) activity in human plasma and cerebrospinal fluid. Biochem Pharmacol 1990; 39: 1353–1356. - PubMed
-
- Erdös EG, Skidgel RA. Neutral endopeptidase 24.11 (enkephalinase) and related regulators of peptide hormones. FASEB J 1989; 3: 145–151. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

