The role of itaconate in host defense and inflammation
- PMID: 35040439
- PMCID: PMC8759771
- DOI: 10.1172/JCI148548
The role of itaconate in host defense and inflammation
Abstract
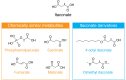
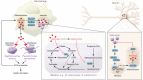
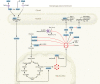
Macrophages exposed to inflammatory stimuli including LPS undergo metabolic reprogramming to facilitate macrophage effector function. This metabolic reprogramming supports phagocytic function, cytokine release, and ROS production that are critical to protective inflammatory responses. The Krebs cycle is a central metabolic pathway within all mammalian cell types. In activated macrophages, distinct breaks in the Krebs cycle regulate macrophage effector function through the accumulation of several metabolites that were recently shown to have signaling roles in immunity. One metabolite that accumulates in macrophages because of the disturbance in the Krebs cycle is itaconate, which is derived from cis-aconitate by the enzyme cis-aconitate decarboxylase (ACOD1), encoded by immunoresponsive gene 1 (Irg1). This Review focuses on itaconate's emergence as a key immunometabolite with diverse roles in immunity and inflammation. These roles include inhibition of succinate dehydrogenase (which controls levels of succinate, a metabolite with multiple roles in inflammation), inhibition of glycolysis at multiple levels (which will limit inflammation), activation of the antiinflammatory transcription factors Nrf2 and ATF3, and inhibition of the NLRP3 inflammasome. Itaconate and its derivatives have antiinflammatory effects in preclinical models of sepsis, viral infections, psoriasis, gout, ischemia/reperfusion injury, and pulmonary fibrosis, pointing to possible itaconate-based therapeutics for a range of inflammatory diseases. This intriguing metabolite continues to yield fascinating insights into the role of metabolic reprogramming in host defense and inflammation.
Conflict of interest statement
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

