Semisupervised Training of a Brain MRI Tumor Detection Model Using Mined Annotations
- PMID: 35040676
- PMCID: PMC8962822
- DOI: 10.1148/radiol.210817
Semisupervised Training of a Brain MRI Tumor Detection Model Using Mined Annotations
Abstract
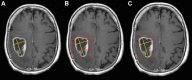
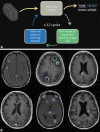
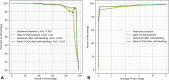
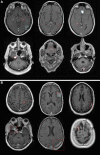
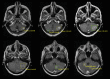
Background Artificial intelligence (AI) applications for cancer imaging conceptually begin with automated tumor detection, which can provide the foundation for downstream AI tasks. However, supervised training requires many image annotations, and performing dedicated post hoc image labeling is burdensome and costly. Purpose To investigate whether clinically generated image annotations can be data mined from the picture archiving and communication system (PACS), automatically curated, and used for semisupervised training of a brain MRI tumor detection model. Materials and Methods In this retrospective study, the cancer center PACS was mined for brain MRI scans acquired between January 2012 and December 2017 and included all annotated axial T1 postcontrast images. Line annotations were converted to boxes, excluding boxes shorter than 1 cm or longer than 7 cm. The resulting boxes were used for supervised training of object detection models using RetinaNet and Mask region-based convolutional neural network (R-CNN) architectures. The best-performing model trained from the mined data set was used to detect unannotated tumors on training images themselves (self-labeling), automatically correcting many of the missing labels. After self-labeling, new models were trained using this expanded data set. Models were scored for precision, recall, and F1 using a held-out test data set comprising 754 manually labeled images from 100 patients (403 intra-axial and 56 extra-axial enhancing tumors). Model F1 scores were compared using bootstrap resampling. Results The PACS query extracted 31 150 line annotations, yielding 11 880 boxes that met inclusion criteria. This mined data set was used to train models, yielding F1 scores of 0.886 for RetinaNet and 0.908 for Mask R-CNN. Self-labeling added 18 562 training boxes, improving model F1 scores to 0.935 (P < .001) and 0.954 (P < .001), respectively. Conclusion The application of semisupervised learning to mined image annotations significantly improved tumor detection performance, achieving an excellent F1 score of 0.954. This development pipeline can be extended for other imaging modalities, repurposing unused data silos to potentially enable automated tumor detection across radiologic modalities. © RSNA, 2022 Online supplemental material is available for this article.
Conflict of interest statement
Figures


References
-
- Tajbakhsh N , Jeyaseelan L , Li Q , Chiang J , Wu Z , Ding X . Embracing Imperfect Datasets: A Review of Deep Learning Solutions for Medical Image Segmentation . Arxiv 1908.10454 [preprint] http://arxiv.org/abs/1908.10454. Posted February 11, 2020. Accessed November 17, 2020 . - PubMed
-
- He K , Gkioxari G , Dollár P , Girshick R . Mask R-CNN . Arxiv 1703.06870 [preprint] http://arxiv.org/abs/1703.06870. Posted January 24, 2018. Accessed November 5, 2019 . - PubMed
-
- Naphade M , Chang MC , Sharma A , et al . The 2018 NVIDIA AI City Challenge. 53–60 . Paper presented at: IEEE Conference on Computer Vision and Pattern Recognition (CVPR) Workshops ; 2018 ; Salt Lake City, Utah . https://openaccess.thecvf.com/content_cvpr_2018_workshops/w3/html/Naphad... Published 2018. Accessed November 12, 2020 .
-
- AlBadawy EA , Saha A , Mazurowski MA . Deep learning for segmentation of brain tumors: Impact of cross-institutional training and testing . Med Phys 2018. ; 45 ( 3 ): 1150 – 1158 . - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

