Elevational Constraints on the Composition and Genomic Attributes of Microbial Communities in Antarctic Soils
- PMID: 35040702
- PMCID: PMC8765064
- DOI: 10.1128/msystems.01330-21
Elevational Constraints on the Composition and Genomic Attributes of Microbial Communities in Antarctic Soils
Abstract
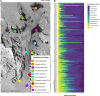
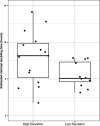
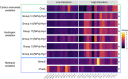
The inland soils found on the Antarctic continent represent one of the more challenging environments for microbial life on Earth. Nevertheless, Antarctic soils harbor unique bacterial and archaeal (prokaryotic) communities able to cope with extremely cold and dry conditions. These communities are not homogeneous, and the taxonomic composition and functional capabilities (genomic attributes) of these communities across environmental gradients remain largely undetermined. We analyzed the prokaryotic communities in soil samples collected from across the Shackleton Glacier region of Antarctica by coupling quantitative PCR, marker gene amplicon sequencing, and shotgun metagenomic sequencing. We found that elevation was the dominant factor explaining differences in the structures of the soil prokaryotic communities, with the drier and saltier soils found at higher elevations harboring less diverse communities and unique assemblages of cooccurring taxa. The higher-elevation soil communities also had lower maximum potential growth rates (as inferred from metagenome-based estimates of codon usage bias) and an overrepresentation of genes associated with trace gas metabolism. Together, these results highlight the utility of assessing community shifts across pronounced environmental gradients to improve our understanding of the microbial diversity found in Antarctic soils and the strategies used by soil microbes to persist at the limits of habitability. IMPORTANCE Antarctic soils represent an ideal system to study how environmental properties shape the taxonomic and functional diversity of microbial communities given the relatively low diversity of Antarctic soil microbial communities and the pronounced environmental gradients that occur across soils located in reasonable proximity to one another. Moreover, the challenging environmental conditions typical of most Antarctic soils present an opportunity to investigate the traits that allow soil microbes to persist in some of the most inhospitable habitats on Earth. We used cultivation-independent methods to study the bacterial and archaeal communities found in soil samples collected from across the Shackleton Glacier region of the Transantarctic Mountains. We show that those environmental characteristics associated with elevation have the greatest impact on the structure of these microbial communities, with the colder, drier, and saltier soils found at higher elevations sustaining less diverse communities that were distinct from those in more hospitable soils with respect to their composition, genomic attributes, and overall life-history strategies. Notably, the harsher conditions found in higher-elevation soils likely select for taxa with lower maximum potential growth rates and an increased reliance on trace gas metabolism to support growth.
Keywords: Antarctica; microbial ecology; soil microbiology; soils.
Conflict of interest statement
The authors declare no conflict of interest.
We declare no competing interests.
Figures


References
-
- Brooks ST, Jabour J, Van Den Hoff J, Bergstrom DM. 2019. Our footprint on Antarctica competes with nature for rare ice-free land. Nat Sustain 2:185–190. doi:10.1038/s41893-019-0237-y. - DOI
-
- Diaz MA, Gardner CB, Welch SA, Jackson WA, Adams BJ, Wall DH, Hogg ID, Fierer N, Lyons WB. 2021. Geochemical zones and environmental gradients for soils from the Central Transantarctic Mountains, Antarctica. Biogeosciences 18:1629–1644. doi:10.5194/bg-18-1629-2021. - DOI
-
- Campbell IB, Claridge GGC. 1987. Antarctica: soils, weathering processes and environment, 1st ed. Elsevier, New York, NY.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
