Cost-utility analysis of apixaban compared with usual care for primary thromboprophylaxis in ambulatory patients with cancer
- PMID: 35040802
- PMCID: PMC8568073
- DOI: 10.1503/cmaj.210523
Cost-utility analysis of apixaban compared with usual care for primary thromboprophylaxis in ambulatory patients with cancer
Abstract
Background: Apixaban (2.5 mg) taken twice daily has been shown to substantially reduce the risk of venous thromboembolism (VTE) compared with placebo for the primary thromboprophylaxis of ambulatory patients with cancer who are starting chemotherapy and are at intermediate-to-high risk of VTE. We aimed to compare the health system costs and health benefits associated with primary thromboprophylaxis using apixaban with those associated with the current standard of care (where no primary thromboprophylaxis is given), from the perspective of Canada's publicly funded health care system in this subpopulation of patients with cancer over a lifetime horizon.
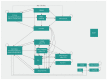
Methods: We performed a cost-utility analysis to estimate the incremental cost per quality-adjusted life-year (QALY) gained with primary thromboprophylaxis using apixaban. We obtained baseline event rates and the efficacy of apixaban from the Apixaban for the Prevention of Venous Thromboembolism in High-Risk Ambulatory Cancer Patients (AVERT) trial on apixaban prophylaxis. We estimated relative risk for bleeding, risk of complications associated with VTE treatment, mortality rates, costs and utilities from other published sources.
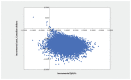
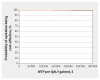
Results: Over a lifetime horizon, apixaban resulted in lower costs to the health system (Can$7902.98 v. Can$14 875.82) and an improvement in QALYs (9.089 v. 9.006). The key driver of cost-effectiveness results was the relative risk of VTE as a result of apixaban. Results from the probabilistic analysis showed that at a willingness to pay of Can$50 000 per QALY, the strategy with the highest probability of being most cost-effective was apixaban, with a probability of 99.87%.
Interpretation: We found that apixaban is a cost-saving option for the primary thromboprophylaxis of ambulatory patients with cancer who are starting chemotherapy and are at intermediate-to-high risk of VTE.
© 2021 CMA Joule Inc. or its licensors.
Conflict of interest statement
Competing interests: Marc Carrier has received consulting fees from Bayer, Sanofi, Servier, BMS, Pfizer, Leo Pharma and Valeo. Philip Wells has received personal fees in the form of payment or honoraria from the Bristol Myers Squibb (BMS)–Pfizer Alliance, Sanofi, Bayer Healthcare and Medscape. He is also the board director for the Bruyère Research Institute and Ottawa Department of Medicine Not-for-Profit Corporation. No other competing interests were declared.
Figures

Comment in
-
Outdated criteria for drug plan reimbursement obstruct evidence-based care.CMAJ. 2021 Oct 12;193(40):E1573-E1574. doi: 10.1503/cmaj.211617. Epub 2021 Oct 11. CMAJ. 2021. PMID: 35040803 Free PMC article. No abstract available.
References
-
- Timp JF, Braekkan SK, Versteeg HH, et al. Epidemiology of cancer-associated venous thrombosis. Blood 2013;122:1712–23. - PubMed
-
- Ay C, Pabinger I, Cohen AT. Cancer-associated venous thromboembolism: burden, mechanisms, and management. Thromb Haemost 2017;117:219–30. - PubMed
-
- Mandalà M, Falanga A, Roila F; ESMO Guidelines Working Group. Management of venous thromboembolism (VTE) in cancer patients: ESMO Clinical Practice Guidelines. Ann Oncol 2011;22(Suppl 6):vi85–92. - PubMed
-
- Key NS, Khorana AA, Kuderer NM, et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: ASCO clinical practice guideline update. J Clin Oncol 2020;38:496–520. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
