Development of a Population Pharmacokinetic Model for the Diroximel Fumarate Metabolites Monomethyl Fumarate and 2-Hydroxyethyl Succinimide Following Oral Administration of Diroximel Fumarate in Healthy Participants and Patients with Multiple Sclerosis
- PMID: 35041178
- PMCID: PMC8857385
- DOI: 10.1007/s40120-021-00316-6
Development of a Population Pharmacokinetic Model for the Diroximel Fumarate Metabolites Monomethyl Fumarate and 2-Hydroxyethyl Succinimide Following Oral Administration of Diroximel Fumarate in Healthy Participants and Patients with Multiple Sclerosis
Abstract
Introduction: Diroximel fumarate (DRF) is a next-generation oral fumarate that is indicated in the USA for relapsing forms of multiple sclerosis (MS). A joint population pharmacokinetic model was developed for the major active metabolite (monomethyl fumarate, MMF) and the major inactive metabolite (2-hydroxyethyl succinimide, HES) of DRF.
Methods: MMF and HES data were included from 341 healthy volunteers and 48 patients with MS across 11 phase I and III studies in which DRF was administered as single or multiple doses. Population modeling was performed with NONMEM version 7.3 with the first-order conditional estimation method.
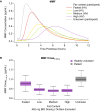
Results: Estimated MMF clearance (CLMMF), volume of distribution, and absorption rate constant (Ka) were 13.5 L/h, 30.4 L, and 5.04 h-1, respectively. CLMMF and HES clearance (CLHES) increased with increasing body weight. CLHES decreased with decreasing renal function. CLMMF and CLHES were 28% and 12% lower in patients with MS than in healthy volunteers, respectively. Ka was reduced in the presence of low-, medium-, and high-fat meals by 37%, 51%, and 67%, respectively, for MMF; and by 34%, 49%, and 62%, respectively, for HES.
Conclusions: Age, sex, race, and baseline liver function parameters such as total bilirubin, albumin, and aspartate aminotransferase were not considered to be significant predictors of MMF or HES disposition.
Keywords: 2-Hydroxyethyl succinimide; Diroximel fumarate; Monomethyl fumarate; Multiple sclerosis; Pharmacokinetics; Population pharmacokinetics.
© 2022. The Author(s).
Figures

References
-
- Biogen: VUMERITY®(diroximel fumarate) delayed-release capsules, for oral use. 2021. https://www.vumerity.com/content/dam/commercial/vumerity/pat/en_us/pdf/v.... Accessed 18 Nov 2021.
-
- Palte MJ, Wehr A, Tawa M, et al. Improving the gastrointestinal tolerability of fumaric acid esters: early findings on gastrointestinal events with diroximel fumarate in patients with relapsing-remitting multiple sclerosis from the phase 3, open-label EVOLVE-MS-1 study. Adv Ther. 2019;36:3154–3165. doi: 10.1007/s12325-019-01085-3. - DOI - PMC - PubMed
-
- Biogen: TECFIDERA® (dimethyl fumarate) delayed-release capsules, for oral use. 2021. https://www.tecfidera.com/content/dam/commercial/tecfidera/pat/en_us/pdf.... Accessed 18 Nov 2021.
-
- Wehr A, Hard M, Yu M, Leigh-Pemberton R, von Moltke L. Relative bioavailability of monomethyl fumarate after administration of ALKS 8700 and dimethyl fumarate in healthy subjects. Neurology. 2018;90:P1.403.
LinkOut - more resources
Full Text Sources

