Cellular crosstalk of glomerular endothelial cells and podocytes in diabetic kidney disease
- PMID: 35041192
- PMCID: PMC9411417
- DOI: 10.1007/s12079-021-00664-w
Cellular crosstalk of glomerular endothelial cells and podocytes in diabetic kidney disease
Abstract
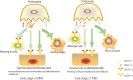
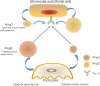
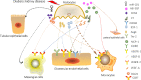
Diabetic kidney disease (DKD) is a serious microvascular complication of diabetes and is the leading cause of end-stage renal disease (ESRD). Persistent proteinuria is an important feature of DKD, which is caused by the destruction of the glomerular filtration barrier (GFB). Glomerular endothelial cells (GECs) and podocytes are important components of the GFB, and their damage can be observed in the early stages of DKD. Recently, studies have found that crosstalk between cells directly affects DKD progression, which has prospective research significance. However, the pathways involved are complex and largely unexplored. Here, we review the literature on cellular crosstalk of GECs and podocytes in the context of DKD, and highlight specific gaps in the field to propose future research directions. Elucidating the intricates of such complex processes will help to further understand the pathogenesis of DKD and develop better prevention and treatment options.
Keywords: Cellular crosstalk; Diabetic kidney disease; Glomerular endothelial cell; High glucose; Podocyte.
© 2022. The International CCN Society.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures


Similar articles
-
Crosstalk among podocytes, glomerular endothelial cells and mesangial cells in diabetic kidney disease: an updated review.Cell Commun Signal. 2024 Feb 19;22(1):136. doi: 10.1186/s12964-024-01502-3. Cell Commun Signal. 2024. PMID: 38374141 Free PMC article. Review.
-
The diabetic microenvironment causes mitochondrial oxidative stress in glomerular endothelial cells and pathological crosstalk with podocytes.Cell Commun Signal. 2020 Jul 8;18(1):105. doi: 10.1186/s12964-020-00605-x. Cell Commun Signal. 2020. PMID: 32641054 Free PMC article.
-
Research progress on exosomes in podocyte injury associated with diabetic kidney disease.Front Endocrinol (Lausanne). 2023 Mar 20;14:1129884. doi: 10.3389/fendo.2023.1129884. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37020588 Free PMC article. Review.
-
Glomerular endothelial cell injury and cross talk in diabetic kidney disease.Am J Physiol Renal Physiol. 2015 Feb 15;308(4):F287-97. doi: 10.1152/ajprenal.00533.2014. Epub 2014 Nov 19. Am J Physiol Renal Physiol. 2015. PMID: 25411387 Free PMC article. Review.
-
Diabetic Kidney Disease, Endothelial Damage, and Podocyte-Endothelial Crosstalk.Kidney Med. 2020 Dec 7;3(1):105-115. doi: 10.1016/j.xkme.2020.10.005. eCollection 2021 Jan-Feb. Kidney Med. 2020. PMID: 33604542 Free PMC article. Review.
Cited by
-
Sirt3 deficiency promotes endothelial dysfunction and aggravates renal injury.PLoS One. 2023 Oct 10;18(10):e0291909. doi: 10.1371/journal.pone.0291909. eCollection 2023. PLoS One. 2023. PMID: 37816025 Free PMC article.
-
Identification of podocyte molecular markers in diabetic kidney disease via single-cell RNA sequencing and machine learning.PLoS One. 2025 Jul 21;20(7):e0328352. doi: 10.1371/journal.pone.0328352. eCollection 2025. PLoS One. 2025. PMID: 40690456 Free PMC article.
-
Cellular crosstalk of mesangial cells and tubular epithelial cells in diabetic kidney disease.Cell Commun Signal. 2023 Oct 16;21(1):288. doi: 10.1186/s12964-023-01323-w. Cell Commun Signal. 2023. PMID: 37845726 Free PMC article. Review.
-
The crosstalk between glomerular endothelial cells and podocytes controls their responses to metabolic stimuli in diabetic nephropathy.Sci Rep. 2023 Oct 20;13(1):17985. doi: 10.1038/s41598-023-45139-7. Sci Rep. 2023. PMID: 37863933 Free PMC article.
-
The Life of a Kidney Podocyte.Acta Physiol (Oxf). 2025 Aug;241(8):e70081. doi: 10.1111/apha.70081. Acta Physiol (Oxf). 2025. PMID: 40698593 Free PMC article. Review.
References
-
- Aly MH, Arafat MA, Hussein OA, Elsaid HH, Abdel-Hammed AR. Study of Angiopoietin-2 and vascular endothelial growth factor as markers of diabetic nephropathy onset in Egyptians diabetic patients with non-albuminuric state. Diabetes Metab Syndr. 2019;13(2):1623–1627. doi: 10.1016/j.dsx.2019.03.016. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

