Selective Estrogen Receptor Modulators Limit Alphavirus Infection by Targeting the Viral Capping Enzyme nsP1
- PMID: 35041501
- PMCID: PMC8923187
- DOI: 10.1128/AAC.01943-21
Selective Estrogen Receptor Modulators Limit Alphavirus Infection by Targeting the Viral Capping Enzyme nsP1
Abstract
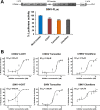
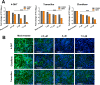
Alphaviruses cause animal or human diseases that are characterized by febrile illness, debilitating arthralgia, or encephalitis. Selective estrogen receptor modulators (SERMs), a class of FDA-approved drugs, have been shown to possess antiviral activities against multiple viruses, including hepatitis C virus, Ebola virus, dengue virus, and vesicular stomatitis virus. Here, we evaluated three SERM compounds, namely, 4-hydroxytamoxifen, tamoxifen, and clomifene, for plausible antiviral properties against two medically important alphaviruses, chikungunya virus (CHIKV) and Sindbis virus (SINV). In cell culture settings, these SERMs displayed potent activity against CHIKV and SINV at nontoxic concentrations with 50% effective concentration (EC50) values ranging between 400 nM and 3.9 μM. Further studies indicated that these compounds inhibit a postentry step of the alphavirus life cycle, while enzymatic assays involving purified recombinant proteins confirmed that these SERMs target the enzymatic activity of nonstructural protein 1 (nsP1), the capping enzyme of alphaviruses. Finally, tamoxifen treatment restrained CHIKV growth in the infected mice and diminished musculoskeletal pathologies. Combining biochemical analyses, cell culture-based studies, and in vivo analyses, we strongly argue that SERM compounds, or their derivatives, may provide for attractive therapeutic options against alphaviruses.
Keywords: SERMs; Sindbis virus; alphavirus; antiviral; chikungunya virus; clomifene; murine model; nsP1; tamoxifen.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Soumahoro M-K, Boelle P-Y, Gaüzere B-A, Atsou K, Pelat C, Lambert B, Ruche GL, Gastellu-Etchegorry M, Renault P, Sarazin M, Yazdanpanah Y, Flahault A, Malvy D, Hanslik T. 2011. The chikungunya epidemic on La Réunion Island in 2005–2006: a cost-of-illness study. PLoS Negl Trop Dis 5:e1197. 10.1371/journal.pntd.0001197. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

