Ultrafast population coding and axo-somatic compartmentalization
- PMID: 35041645
- PMCID: PMC8797191
- DOI: 10.1371/journal.pcbi.1009775
Ultrafast population coding and axo-somatic compartmentalization
Abstract
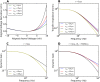
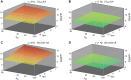
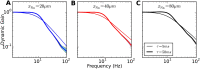
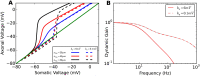
Populations of cortical neurons respond to common input within a millisecond. Morphological features and active ion channel properties were suggested to contribute to this astonishing processing speed. Here we report an exhaustive study of ultrafast population coding for varying axon initial segment (AIS) location, soma size, and axonal current properties. In particular, we studied their impact on two experimentally observed features 1) precise action potential timing, manifested in a wide-bandwidth dynamic gain, and 2) high-frequency boost under slowly fluctuating correlated input. While the density of axonal channels and their distance from the soma had a very small impact on bandwidth, it could be moderately improved by increasing soma size. When the voltage sensitivity of axonal currents was increased we observed ultrafast coding and high-frequency boost. We conclude that these computationally relevant features are strongly dependent on axonal ion channels' voltage sensitivity, but not their number or exact location. We point out that ion channel properties, unlike dendrite size, can undergo rapid physiological modification, suggesting that the temporal accuracy of neuronal population encoding could be dynamically regulated. Our results are in line with recent experimental findings in AIS pathologies and establish a framework to study structure-function relations in AIS molecular design.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

