C-type Natriuretic Peptide-induced PKA Activation Promotes Endochondral Bone Formation in Hypertrophic Chondrocytes
- PMID: 35041746
- PMCID: PMC8826897
- DOI: 10.1210/endocr/bqac005
C-type Natriuretic Peptide-induced PKA Activation Promotes Endochondral Bone Formation in Hypertrophic Chondrocytes
Abstract
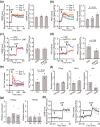
Longitudinal bone growth is achieved by a tightly controlled process termed endochondral bone formation. C-type natriuretic peptide (CNP) stimulates endochondral bone formation through binding to its specific receptor, guanylyl cyclase (GC)-B. However, CNP/GC-B signaling dynamics in different stages of endochondral bone formation have not been fully clarified, especially in terms of the interaction between the cyclic guanine monophosphate (cGMP) and cyclic adenosine monophosphate (cAMP) pathways. Here, we demonstrated that CNP activates the cAMP/protein kinase A (PKA) pathway and that this activation contributed to the elongation of the hypertrophic zone in the growth plate. Cells of the chondrogenic line ATDC5 were transfected with Förster resonance energy transfer (FRET)-based cGMP and PKA biosensors. Dual-FRET imaging revealed that CNP increased intracellular cGMP levels and PKA activities in chondrocytes. Further, CNP-induced PKA activation was enhanced following differentiation of ATDC5 cells. Live imaging of the fetal growth plate of transgenic mice, expressing a FRET biosensor for PKA, PKAchu mice, showed that CNP predominantly activates the PKA in the hypertrophic chondrocytes. Additionally, histological analysis of the growth plate of PKAchu mice demonstrated that CNP increased the length of the growth plate, but coadministration of a PKA inhibitor, H89, inhibited the growth-promoting effect of CNP only in the hypertrophic zone. In summary, we revealed that CNP-induced cGMP elevation activated the cAMP/PKA pathway, and clarified that this PKA activation contributed to the bone growth-promoting effect of CNP in hypertrophic chondrocytes. These results provide insights regarding the cross-talk between cGMP and cAMP signaling in endochondral bone formation and in the physiological role of the CNP/GC-B system.
Keywords: C-type natriuretic peptide; FRET biosensor; endochondral bone formation; live imaging; protein kinase A.
© The Author(s) 2022. Published by Oxford University Press on behalf of the Endocrine Society.
Figures




References
-
- Kronenberg HM. Developmental regulation of the growth plate. Nature. 2003;423(6937):332-336. - PubMed
-
- Nakao K, Ogawa Y, Suga S, Imura H. Molecular biology and biochemistry of the natriuretic peptide system. II: natriuretic peptide receptors. J Hypertens. 1992;10(10):1111-1114. - PubMed
-
- Yasoda A, Ogawa Y, Suda M, et al. . Natriuretic peptide regulation of endochondral ossification. Evidence for possible roles of the C-type natriuretic peptide/guanylyl cyclase-B pathway. J Biol Chem. 1998;273(19):11695-11700. - PubMed
-
- Yasoda A, Komatsu Y, Chusho H, et al. . Overexpression of CNP in chondrocytes rescues achondroplasia through a MAPK-dependent pathway. Nat Med. 2004;10(1):80-86. - PubMed

