Real-World Experience of Cryopreserved Allogeneic Hematopoietic Grafts during the COVID-19 Pandemic: A Single-Center Report
- PMID: 35042013
- PMCID: PMC8760704
- DOI: 10.1016/j.jtct.2022.01.010
Real-World Experience of Cryopreserved Allogeneic Hematopoietic Grafts during the COVID-19 Pandemic: A Single-Center Report
Abstract
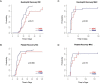
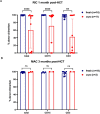
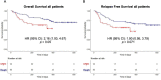
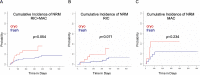
In response to the widespread COVID-19 pandemic, cryopreservation of allogeneic donor apheresis products was implemented to mitigate the challenges of donor availability and product transport. Although logistically beneficial, the impact of cryopreservation on clinical outcomes and graft composition remains unclear. In this study, we compared outcomes and graft composition with cryopreserved versus fresh allografts in the setting of allogeneic hematopoietic cell transplantation (allo-HCT). We retrospectively analyzed the clinical outcomes of 30 consecutive patients who received cryopreserved allografts between March and August 2020 and 60 consecutive patients who received fresh allografts before the COVID-19 pandemic. Primary endpoints were hematopoietic engraftment and graft failure (GF), and secondary outcomes were overall survival (OS), relapse-free survival (RFS) and nonrelapse mortality (NRM). In addition, extended immunophenotype analysis was performed on cryopreserved and prospectively collected fresh apheresis samples. Compared with recipients of fresh allografts, both neutrophil and platelet recovery were delayed in recipients of cryopreserved reduced-intensity conditioning (RIC) allo-HCT, with a median time to engraftment of 24 days versus 18 days (P = .01) for neutrophils and 27 days versus 18 days (P = .069) for platelets. We observed primary GF in 4 of 30 patients in the cryopreserved cohort (13.3%) versus only 1 of 60 patients (1.7 %) in the fresh cohort (P = .03). Cryopreserved RIC allo-HCT was associated with significantly lower median total, myeloid, and T cell donor chimerism at 1 month. OS and RFS were inferior for cryopreserved graft recipients (hazard ratio [HR], 2.16; 95% confidence interval [CI], 1.00 to 4.67) and HR, 1.90; 95% CI, 0.95 to 3.79, respectively. Using an extended immunophenotype analysis, we compared 14 samples from the cryopreserved cohort to 6 prospectively collected fresh apheresis donor samples. These analyses showed both a decrease in total cell viability and a significantly reduced absolute number of natural killer cells (CD3-CD56+) in the cryopreserved apheresis samples. In this single-institution study, we found delayed engraftment and a trend toward clinical inferiority of cryopreserved allografts compared with fresh allografts. Further evaluation of the use of cryopreserved allografts and their impact on clinical and laboratory outcomes is warranted.
Keywords: Cryopreserved allografts; Engraftment failure; Graft composition; Reduced-intensity conditioning.
Copyright © 2022. Published by Elsevier Inc.
Figures

Similar articles
-
Universal Engraftment after Allogeneic Hematopoietic Cell Transplantation Using Cryopreserved CD34-Selected Grafts.Transplant Cell Ther. 2021 Aug;27(8):697.e1-697.e5. doi: 10.1016/j.jtct.2021.04.026. Epub 2021 May 13. Transplant Cell Ther. 2021. PMID: 33991721 Free PMC article.
-
Graft Cryopreservation Does Not Impact Overall Survival after Allogeneic Hematopoietic Cell Transplantation Using Post-Transplantation Cyclophosphamide for Graft-versus-Host Disease Prophylaxis.Biol Blood Marrow Transplant. 2020 Jul;26(7):1312-1317. doi: 10.1016/j.bbmt.2020.04.001. Epub 2020 Apr 10. Biol Blood Marrow Transplant. 2020. PMID: 32283185 Free PMC article.
-
The Effect of Donor Graft Cryopreservation on Allogeneic Hematopoietic Cell Transplantation Outcomes: A Center for International Blood and Marrow Transplant Research Analysis. Implications during the COVID-19 Pandemic.Transplant Cell Ther. 2021 Jun;27(6):507-516. doi: 10.1016/j.jtct.2021.03.015. Epub 2021 Mar 22. Transplant Cell Ther. 2021. PMID: 33865804 Free PMC article.
-
The effect of cryopreservation on engraftment kinetics in fully matched allogeneic stem cell transplantation: Real-life data and literature review.Transfus Apher Sci. 2023 Dec;62(6):103821. doi: 10.1016/j.transci.2023.103821. Epub 2023 Sep 18. Transfus Apher Sci. 2023. PMID: 37775358 Review.
-
Has allogeneic stem cell cryopreservation been given the 'cold shoulder'? An analysis of the pros and cons of using frozen versus fresh stem cell products in allogeneic stem cell transplantation.Bone Marrow Transplant. 2006 Sep;38(6):399-405. doi: 10.1038/sj.bmt.1705462. Epub 2006 Aug 7. Bone Marrow Transplant. 2006. PMID: 16892075 Review.
Cited by
-
Pros and Cons of Cryopreserving Allogeneic Stem Cell Products.Cells. 2024 Mar 21;13(6):552. doi: 10.3390/cells13060552. Cells. 2024. PMID: 38534396 Free PMC article. Review.
-
Characterization and Function of Cryopreserved Bone Marrow from Deceased Organ Donors: A Potential Viable Alternative Graft Source.Transplant Cell Ther. 2023 Feb;29(2):95.e1-95.e10. doi: 10.1016/j.jtct.2022.11.010. Epub 2022 Nov 17. Transplant Cell Ther. 2023. PMID: 36402456 Free PMC article.
-
Allogeneic hematopoietic stem cell transplantation in the COVID-19 era.Front Immunol. 2023 Feb 22;14:1100468. doi: 10.3389/fimmu.2023.1100468. eCollection 2023. Front Immunol. 2023. PMID: 36911678 Free PMC article. Review.
-
Clinical impact of cryopreservation of allogeneic hematopoietic cell grafts during the onset of the COVID-19 pandemic.Blood Adv. 2023 Oct 10;7(19):5982-5993. doi: 10.1182/bloodadvances.2023009786. Blood Adv. 2023. PMID: 37036959 Free PMC article.
-
Clinical Impact of Graft Cryopreservation on Allogeneic Stem Cell Transplantation: An Italian, Registry-Based Study on Behalf of the "Gruppo Italiano Per Il Trapianto di Midollo Osseo, Cellule Staminali Emopoietiche e Terapia Cellulare" (GITMO).Am J Hematol. 2025 Aug;100(8):1354-1364. doi: 10.1002/ajh.27731. Epub 2025 Jun 2. Am J Hematol. 2025. PMID: 40456127 Free PMC article.
References
-
- Frey NV, Lazarus HM, Goldstein SC. Has allogeneic stem cell cryopreservation been given the “cold shoulder”? An analysis of the pros and cons of using frozen versus fresh stem cell products in allogeneic stem cell transplantation. Bone Marrow Transplant. 2006;38:399–405. - PubMed
-
- Kim DH, Jamal N, Saragosa R, et al. Similar outcomes of cryopreserved allogeneic peripheral stem cell transplants (PBSCT) compared to fresh allografts. Biol Blood Marrow Transplant. 2007;13:1233–1243. - PubMed
-
- Lioznov M, Dellbrugger C, Sputtek A, Fehse B, Kröger N, Zander AR. Transportation and cryopreservation may impair haematopoietic stem cell function and engraftment of allogeneic PBSCs, but not BM. Bone Marrow Transplant. 2008;42:121–128. - PubMed
-
- Medd P, Nagra S, Hollyman D, Craddock C, Malladi R. Cryopreservation of allogeneic PBSC from related and unrelated donors is associated with delayed platelet engraftment but has no impact on survival. Bone Marrow Transplant. 2013;48:243–248. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

