Cell therapy in vascularized composite allotransplantation
- PMID: 35042019
- PMCID: PMC9422067
- DOI: 10.1016/j.bj.2022.01.005
Cell therapy in vascularized composite allotransplantation
Abstract
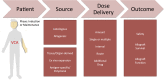
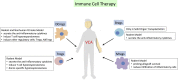
Allograft rejection is one of the obstacles in achieving a successful vascularized composite allotransplantation (VCA). Treatments of graft rejection with lifelong immunosuppression (IS) subject the recipients to a lifelong risk of cancer development and opportunistic infections. Cell therapy has recently emerged as a promising strategy to modulate the immune system, minimize immunosuppressant drug dosages, and induce allograft tolerance. In this review, the recent works regarding the use of cell therapy to improve allograft outcomes are discussed. The current data supports the safety of cell therapy. The suitable type of cell therapy in allotransplantation is clinically dependent. Bone marrow cell therapy is more suitable for the induction phase, while other cell therapies are more feasible in either the induction or maintenance phase, or for salvage of allograft rejection. Immune cell therapy focuses on modulating the immune response, whereas stem cells may have an additional role in promoting structural regenerations, such as nerve regeneration. Source, frequency, dosage, and route of cell therapy delivery are also dependent on the specific need in the clinical setting.
Keywords: Cell therapy; Clinical transplantation; Immune cell; Stem cell; Vascularized composite allotransplantation.
Copyright © 2022 Chang Gung University. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Conflicts of interest The authors declare no conflicts of interest.
Figures


References
-
- Broyles J.M., Alrakan M., Ensor C.R., Khalifian S., Kotton C.N., Avery R.K., et al. Characterization, prophylaxis, and treatment of infectious complications in craniomaxillofacial and upper extremity allotransplantation: a multicenter perspective. Plast Reconstr Surg. 2014;133:543e–551e. - PubMed
-
- Coffman K.L., Siemionow M.Z. Ethics of facial transplantation revisited. Curr Opin Organ Transplant. 2014;19:181–187. - PubMed
-
- Siemionow M.Z., Zor F., Gordon C.R. Face, upper extremity, and concomitant transplantation: potential concerns and challenges ahead. Plast Reconstr Surg. 2010;126:308–315. - PubMed
-
- Petruzzo P., Dubernard J.M. The international registry on hand and composite tissue allotransplantation. Clin Transpl. 2011:247–253. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

