Final efficacy and safety data, and exploratory molecular profiling from the phase III ALUR study of alectinib versus chemotherapy in crizotinib-pretreated ALK-positive non-small-cell lung cancer
- PMID: 35042152
- PMCID: PMC8777286
- DOI: 10.1016/j.esmoop.2021.100333
Final efficacy and safety data, and exploratory molecular profiling from the phase III ALUR study of alectinib versus chemotherapy in crizotinib-pretreated ALK-positive non-small-cell lung cancer
Abstract
Background: At the primary data cut-off, the ALUR study demonstrated significantly improved progression-free survival (PFS) and central nervous system (CNS) objective response rate (ORR) with alectinib versus chemotherapy in pretreated, advanced anaplastic lymphoma kinase (ALK)-positive non-small-cell lung cancer. We report final efficacy and safety data, and exploratory molecular profiling.
Patients and methods: Patients who received prior platinum-doublet chemotherapy and crizotinib were randomized 2 : 1 to receive alectinib 600 mg twice daily (n = 79) or chemotherapy (pemetrexed 500 mg/m2 or docetaxel 75 mg/m2, every 3 weeks; n = 40) until progressive disease, death or withdrawal. The primary endpoint was investigator-assessed PFS. Secondary endpoints included ORR, CNS ORR and safety. Plasma samples were collected at baseline, then every 6 weeks until progressive disease; molecular factors detected by next-generation sequencing were correlated with outcomes.
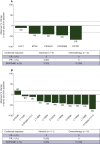
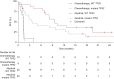
Results: Investigator-assessed PFS was significantly longer with alectinib than chemotherapy (median 10.9 versus 1.4 months; hazard ratio 0.20, 95% confidence interval 0.12-0.33; P < 0.001). ORR was 50.6% with alectinib versus 2.5% with chemotherapy (P < 0.001). In patients with measurable CNS metastases at baseline, CNS ORR was 66.7% with alectinib versus 0% with chemotherapy (P < 0.001). No new safety signals were seen. ALK rearrangement was identified in 69.5% (n = 41/59) of baseline plasma samples. Confirmed partial responses were observed with alectinib in 6/11 patients with a secondary ALK mutation and 4/6 patients with a non-EML4-ALK (where EML4 is echinoderm microtubule-associated protein-like 4) fusion. Detection of mutant TP53 in baseline plasma resulted in numerically shorter PFS with alectinib (hazard ratio 1.88, 95% confidence interval 0.9-3.93).
Conclusions: Final efficacy data from ALUR confirmed the superior PFS, ORR and CNS ORR of alectinib versus chemotherapy in pretreated, advanced ALK-positive non-small-cell lung cancer. Alectinib prolonged PFS versus chemotherapy in patients with wild-type or mutant TP53; however, alectinib activity was considerably decreased in patients with mutant TP53.
Keywords: ALK-positive NSCLC; ALUR; TP53; alectinib; chemotherapy.
Copyright © 2021 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Disclosure JW has received personal fees and/or grants from Amgen, AstraZeneca (AZ), Bayer, Blueprint, Boehringer Ingelheim (BI), Bristol-Myers Squibb (BMS), Chugai, Daiichi Sankyo, Ignyta, Janssen, Lilly, Loxo, Merck Sharp & Dohme (MSD), Novartis, Pfizer, Roche, Seattle Genetics and Takeda. ÅH has received personal fees and/or grants from AZ, BMS, MSD, Pfizer, Pierre-Fabre, Roche and Takeda. I-JO has received personal fees and/or grants from AZ, BI, BMS, Menarini, MSD, ONO, Pfizer, Roche and Takeda. MRM has received personal fees from AZ, BI, BMS, MSD and Roche. RD has received honoraria or consulting fees from AZ, BI, Clovis Oncology, Novartis, Pfizer, Roche, Foundation Medicine, MSD, Seattle Genetics and Takeda. AW has received travel, accommodation, or expenses from, and participated in speakers' bureaus for BMS, Pfizer, Takeda and Roche. J de C has received personal fees from Amgen, AZ, BI, BMS, Eli Lilly, MSD, Novartis, Pfizer, Roche and Takeda. JM has received personal fees from AZ, BMS, MSD, Novartis, Pfizer and Roche; and grants from AZ and Roche. FG has received personal fees and/or grants from AZ, BI, BMS, Celgene, Chugai, Lilly, MSD, Novartis, Pfizer, Roche and Takeda. MC, AC, TR, KT and VS are employees of F. Hoffmann-La Roche Ltd. TR and KT also own Roche stocks. SN has received personal fees from AMG, AZ, BMS, Eli Lilly, MSD and Roche, and participated in speakers' bureaus for BeiGene, Pfizer, Sanofi and Takeda. Data sharing Qualified researchers may request access to individual patient level data through the clinical study data request platform (https://vivli.org/). Further details on Roche's criteria for eligible studies are available here (https://vivli.org/members/ourmembers/). For further details on Roche's Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, see here (https://www.roche.com/research_and_development/who_we_are_how_we_work/clinical_trials/our_commitment_to_data_sharing.htm).
Figures

References
-
- Barlesi F., Mazieres J., Merlio J.P., et al. Routine molecular profiling of patients with advanced non-small-cell lung cancer: results of a 1-year nationwide programme of the French Cooperative Thoracic Intergroup (IFCT) Lancet. 2016;387(10026):1415–1426. - PubMed
-
- Tian H.-X., Zhang X.-C., Yang J.-J., et al. Clinical characteristics and sequence complexity of anaplastic lymphoma kinase gene fusions in Chinese lung cancer patients. Lung Cancer. 2017;114:90–95. - PubMed
-
- US Food & Drug Administration (FDA) Alectinib Prescribing Information (PI) 2021. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/208434s004lbl.pdf Available at.
-
- European Medicines Agency (EMA) Alectinib Summary of Product Characteristics (SmPC) https://www.ema.europa.eu/en/documents/product-information/alecensa-epar... Available at.
-
- Hanna N.H., Robinson A.G., Temin S., et al. Therapy for stage IV non-small-cell lung cancer with driver alterations: ASCO and OH (CCO) Joint Guideline Update. J Clin Oncol. 2021;39(9):1040–1091. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

