Molecular accuracy vs antigenic speed: SARS-CoV-2 testing strategies
- PMID: 35042168
- PMCID: PMC8687762
- DOI: 10.1016/j.coph.2021.12.006
Molecular accuracy vs antigenic speed: SARS-CoV-2 testing strategies
Abstract
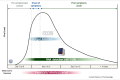
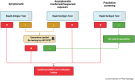
The pandemic caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has hit every corner of the world faster than any infectious disease ever known. In this context, rapid and accurate testing of positive cases are essential to follow the test-trace-isolate strategy (TETRIS), which has proven to be a key approach to constrain viral spread. Here, we discuss how to interpret and combine molecular or/and antigen-based detection methods for SARS-CoV-2 as well as when they should be used. Their application can be cleverly designed as an algorithm to prevent viral dissemination according to distinct epidemiological contexts within surveillance programs.
Keywords: Antigenic test; RT-PCR; SARS-CoV-2; Testing strategies.
Copyright © 2022. Published by Elsevier Ltd.
Figures
Similar articles
-
[Evaluation of the Rapid Antigen Detection Kit with the Polymerase Chain Reaction for Detection of SARS-CoV-2 in Respiratory Samples].Mikrobiyol Bul. 2022 Apr;56(2):263-273. doi: 10.5578/mb.20229806. Mikrobiyol Bul. 2022. PMID: 35477229 Turkish.
-
[Retrospective analysis of the performance of the SARS-CoV-2 rapid antigen detection test compared to the reference RT-PCR test].Ann Biol Clin (Paris). 2021 Apr 1;79(2):168-175. doi: 10.1684/abc.2021.1641. Ann Biol Clin (Paris). 2021. PMID: 33985935 French.
-
Effectiveness and cost-effectiveness of four different strategies for SARS-CoV-2 surveillance in the general population (CoV-Surv Study): a structured summary of a study protocol for a cluster-randomised, two-factorial controlled trial.Trials. 2021 Jan 8;22(1):39. doi: 10.1186/s13063-020-04982-z. Trials. 2021. PMID: 33419461 Free PMC article.
-
[SARS-CoV-2 and Microbiological Diagnostic Dynamics in COVID-19 Pandemic].Mikrobiyol Bul. 2020 Jul;54(3):497-509. doi: 10.5578/mb.69839. Mikrobiyol Bul. 2020. PMID: 32755524 Review. Turkish.
-
Immunologic Testing for SARS-CoV-2 Infection from the Antigen Perspective.J Clin Microbiol. 2021 Apr 20;59(5):e02160-20. doi: 10.1128/JCM.02160-20. Print 2021 Apr 20. J Clin Microbiol. 2021. PMID: 33318065 Free PMC article. Review.
Cited by
-
Diagnosis of COVID-19. What have we learned after two years of the pandemic?Adv Lab Med. 2022 Jun 15;3(2):97-102. doi: 10.1515/almed-2022-0041. eCollection 2022 Jun. Adv Lab Med. 2022. PMID: 37361865 Free PMC article. No abstract available.
-
Clinical and molecular fingerprint of SARS-CoV-2 among hospital employees in a period of Omicron BA.2 dominance.GMS Hyg Infect Control. 2025 Feb 24;20:Doc02. doi: 10.3205/dgkh000531. eCollection 2025. GMS Hyg Infect Control. 2025. PMID: 40352653 Free PMC article.
-
Real-world usage of mass rapid antigen testing for COVID-19 in long-term care facilities and support programmes: results from long-term surveillance in North-Eastern Germany.BMC Public Health. 2025 May 15;25(1):1785. doi: 10.1186/s12889-025-22914-x. BMC Public Health. 2025. PMID: 40375166 Free PMC article.
-
Comparison of extraction-based and elution-based polymerase chain reaction testing, and automated and rapid antigen testing for the diagnosis of severe acute respiratory syndrome coronavirus 2.J Med Virol. 2022 Jul;94(7):3155-3159. doi: 10.1002/jmv.27709. Epub 2022 Mar 22. J Med Virol. 2022. PMID: 35274327 Free PMC article.
References
-
- Moreno P., Moratorio G., Iraola G., Fajardo Á., Aldunate F., Pereira-Gómez M., Perbolianachis P., Costábile A., López-Tort F., Simón D., et al. An effective COVID-19 response in South America: the Uruguayan Conundrum. medRxiv. 2020 doi: 10.1101/2020.07.24.20161802. - DOI
-
- Chung S.-C., Marlow S., Tobias N., Alogna A., Alogna I., You S.-L., Khunti K., McKee M., Michie S., Pillay D. Original research: lessons from countries implementing find, test, trace, isolation and support policies in the rapid response of the COVID-19 pandemic: a systematic review. BMJ Open. 2021;11 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

