Analysis of preplatelets and their barbell platelet derivatives by imaging flow cytometry
- PMID: 35042240
- PMCID: PMC9092408
- DOI: 10.1182/bloodadvances.2021006073
Analysis of preplatelets and their barbell platelet derivatives by imaging flow cytometry
Abstract
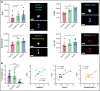
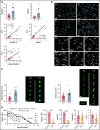
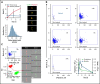
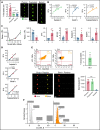
Circulating large "preplatelets" undergo fission via barbell platelet intermediates into two smaller, mature platelets. In this study, we determine whether preplatelets and/or barbells are equivalent to reticulated/immature platelets by using ImageStream flow cytometry and super-resolution microscopy. Immature platelets, preplatelets, and barbells were quantified in healthy and thrombocytopenic mice, healthy human volunteers, and patients with immune thrombocytopenia or undergoing chemotherapy. Preplatelets and barbells were 1.9% ± 0.18%/1.7% ± 0.48% (n = 6) and 3.3% ± 1.6%/0.5% ± 0.27% (n = 12) of total platelet counts in murine and human whole blood, respectively. Both preplatelets and barbells exhibited high expression of major histocompatibility complex class I with high thiazole orange and Mitotracker fluorescence. Tracking dye experiments confirmed that preplatelets transform into barbells and undergo fission ex vivo to increase platelet counts, with dependence on the cytoskeleton and normal mitochondrial respiration. Samples from antibody-induced thrombocytopenia in mice and patients with immune thrombocytopenia had increased levels of both preplatelets and barbells correlating with immature platelet levels. Furthermore, barbells were absent after chemotherapy in patients. In mice, in vivo biotinylation confirmed that barbells, but not all large platelets, were immature. This study demonstrates that a subpopulation of large platelets are immature preplatelets that can transform into barbells and undergo fission during maturation.
© 2022 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Figures




References
-
- Ault KA, Knowles C. In vivo biotinylation demonstrates that reticulated platelets are the youngest platelets in circulation. Exp Hematol. 1995;23(9):996-1001. - PubMed
-
- Ingram M, Coopersmith A. Reticulated platelets following acute blood loss. Br J Haematol. 1969;17(3):225-229. - PubMed
-
- Kienast J, Schmitz G. Flow cytometric analysis of thiazole orange uptake by platelets: a diagnostic aid in the evaluation of thrombocytopenic disorders. Blood. 1990;75(1):116-121. - PubMed

