Extraordinarily precise nematode sex ratios: adaptive responses to vanishingly rare mating opportunities
- PMID: 35042409
- PMCID: PMC8767218
- DOI: 10.1098/rspb.2021.1572
Extraordinarily precise nematode sex ratios: adaptive responses to vanishingly rare mating opportunities
Abstract
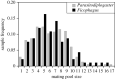
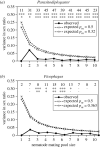
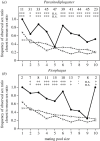
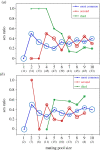
Sex ratio theory predicts both mean sex ratio and variance under a range of population structures. Here, we compare two genera of phoretic nematodes (Parasitodiplogaster and Ficophagus spp.) associated with 12 fig pollinating wasp species in Panama. The host wasps exhibit classic local mate competition: only inseminated females disperse from natal figs, and their offspring form mating pools that consist of scores of the adult offspring contributed by one or a few foundress mothers. By contrast, in both nematode genera, only sexually undifferentiated juveniles disperse and their mating pools routinely consist of 10 or fewer adults. Across all mating pool sizes, the sex ratios observed in both nematode genera are consistently female-biased (approx. 0.34 males), but markedly less female-biased than is often observed in the host wasps (approx. 0.10 males). In further contrast with their hosts, variances in nematode sex ratios are also consistently precise (significantly less than binomial). The constraints associated with predictably small mating pools within highly subdivided populations appear to select for precise sex ratios that contribute both to the reproductive success of individual nematodes, and to the evolutionary persistence of nematode species. We suggest that some form of environmental sex determination underlies these precise sex ratios.
Keywords: Ficophagus; Ficus; Parasitodiplogaster; environmental sex determination; sex allocation; sex ratio variance.
Figures

References
-
- Darwin C. 1871. The descent of man and selection in relation to sex, 1st edn. London, UK: John Murray.
-
- Fisher RA. 1930. The genetical theory of natural selection. Oxford, UK: The Clarendon Press.
-
- Darwin C. 1882. The descent of man and selection in relation to sex, 2nd edn. London, UK: John Murray.
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Miscellaneous
