Radiotherapy transiently reduces the sensitivity of cancer cells to lymphocyte cytotoxicity
- PMID: 35042775
- PMCID: PMC8785960
- DOI: 10.1073/pnas.2111900119
Radiotherapy transiently reduces the sensitivity of cancer cells to lymphocyte cytotoxicity
Abstract
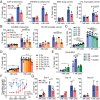
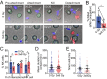
The impact of radiotherapy on the interaction between immune cells and cancer cells is important not least because radiotherapy can be used alongside immunotherapy as a cancer treatment. Unexpectedly, we found that X-ray irradiation of cancer cells induced significant resistance to natural killer (NK) cell killing. This was true across a wide variety of cancer-cell types as well as for antibody-dependent cellular cytotoxicity. Resistance appeared 72 h postirradiation and persisted for 2 wk. Resistance could also occur independently of radiotherapy through pharmacologically induced cell-cycle arrest. Crucially, multiple steps in NK-cell engagement, synapse assembly, and activation were unaffected by target cell irradiation. Instead, radiotherapy caused profound resistance to perforin-induced calcium flux and lysis. Resistance also occurred to a structurally similar bacterial toxin, streptolysin O. Radiotherapy did not affect the binding of pore-forming proteins at the cell surface or membrane repair. Rather, irradiation instigated a defect in functional pore formation, consistent with phosphatidylserine-mediated perforin inhibition. In vivo, radiotherapy also led to a significant reduction in NK cell-mediated clearance of cancer cells. Radiotherapy-induced resistance to perforin also constrained chimeric antigen receptor T-cell cytotoxicity. Together, these data establish a treatment-induced resistance to lymphocyte cytotoxicity that is important to consider in the design of radiotherapy-immunotherapy protocols.
Keywords: NK cell; T cell; cancer; perforin; radiotherapy.
Copyright © 2022 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no competing interest.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

