Plasmodium falciparum genomic surveillance reveals spatial and temporal trends, association of genetic and physical distance, and household clustering
- PMID: 35042879
- PMCID: PMC8766587
- DOI: 10.1038/s41598-021-04572-2
Plasmodium falciparum genomic surveillance reveals spatial and temporal trends, association of genetic and physical distance, and household clustering
Abstract
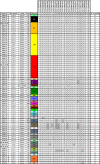
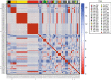
Molecular epidemiology using genomic data can help identify relationships between malaria parasite population structure, malaria transmission intensity, and ultimately help generate actionable data to assess the effectiveness of malaria control strategies. Genomic data, coupled with geographic information systems data, can further identify clusters or hotspots of malaria transmission, parasite genetic and spatial connectivity, and parasite movement by human or mosquito mobility over time and space. In this study, we performed longitudinal genomic surveillance in a cohort of 70 participants over four years from different neighborhoods and households in Thiès, Senegal-a region of exceptionally low malaria transmission (entomological inoculation rate less than 1). Genetic identity (identity by state, IBS) was established using a 24-single nucleotide polymorphism molecular barcode, identity by descent was calculated from whole genome sequence data, and a hierarchical Bayesian regression model was used to establish genetic and spatial relationships. Our results show clustering of genetically similar parasites within households and a decline in genetic similarity of parasites with increasing distance. One household showed extremely high diversity and warrants further investigation as to the source of these diverse genetic types. This study illustrates the utility of genomic data with traditional epidemiological approaches for surveillance and detection of trends and patterns in malaria transmission not only by neighborhood but also by household. This approach can be implemented regionally and countrywide to strengthen and support malaria control and elimination efforts.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- World Malaria Report 2020: 20 years of global progress and challenges. Report, World Health Organization (2020).
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

