Bacteriophage EK99P-1 alleviates enterotoxigenic Escherichia coli K99-induced barrier dysfunction and inflammation
- PMID: 35042907
- PMCID: PMC8766502
- DOI: 10.1038/s41598-022-04861-4
Bacteriophage EK99P-1 alleviates enterotoxigenic Escherichia coli K99-induced barrier dysfunction and inflammation
Abstract
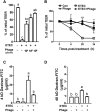
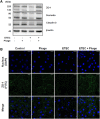
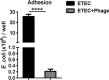
Bacteriophages, simply phages, have long been used as a potential alternative to antibiotics for livestock due to their ability to specifically kill enterotoxigenic Escherichia coli (ETEC), which is a major cause of diarrhea in piglets. However, the control of ETEC infection by phages within intestinal epithelial cells, and their relationship with host immune responses, remain poorly understood. In this study, we evaluated the effect of phage EK99P-1 against ETEC K99-infected porcine intestinal epithelial cell line (IPEC-J2). Phage EK99P-1 prevented ETEC K99-induced barrier disruption by attenuating the increased permeability mediated by the loss of tight junction proteins such as zonula occludens-1 (ZO-1), occludin, and claudin-3. ETEC K99-induced inflammatory responses, such as interleukin (IL)-8 secretion, were decreased by treatment with phage EK99P-1. We used a IPEC-J2/peripheral blood mononuclear cell (PBMC) transwell co-culture system to investigate whether the modulation of barrier disruption and chemokine secretion by phage EK99P-1 in ETEC K99-infected IPEC-J2 would influence immune cells at the site of basolateral. The results showed that phage EK99P-1 reduced the mRNA expression of ETEC K99-induced pro-inflammatory cytokines, IL-1β and IL-8, from PBMC collected on the basolateral side. Together, these results suggest that phage EK99P-1 prevented ETEC K99-induced barrier dysfunction in IPEC-J2 and alleviated inflammation caused by ETEC K99 infection. Reinforcement of the intestinal barrier, such as regulation of permeability and cytokines, by phage EK99P-1 also modulates the immune cell inflammatory response.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
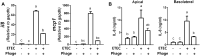
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

