This is a preprint.
A single dose ChAdOx1 nCoV-19 vaccine elicits high antibody responses in individuals with prior SARS-CoV-2 infection comparable to that of double dose vaccinated SARS-CoV-2 infection naïve individuals
- PMID: 35043108
- PMCID: PMC8764722
- DOI: 10.21203/rs.3.rs-1250175/v1
A single dose ChAdOx1 nCoV-19 vaccine elicits high antibody responses in individuals with prior SARS-CoV-2 infection comparable to that of double dose vaccinated SARS-CoV-2 infection naïve individuals
Update in
-
A Single Dose of ChAdOx1 nCoV-19 Vaccine Elicits High Antibody Responses in Individuals with Prior SARS-CoV-2 Infection Comparable to That of Two-Dose-Vaccinated, SARS-CoV-2-Infection-Naïve Individuals: A Longitudinal Study in Ethiopian Health Workers.Vaccines (Basel). 2022 May 27;10(6):859. doi: 10.3390/vaccines10060859. Vaccines (Basel). 2022. PMID: 35746467 Free PMC article.
Abstract
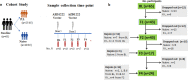
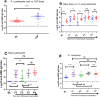
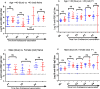
Background A single dose COVID-19 vaccines, mostly mRNA-based vaccines, are shown to induce robust antibody responses in individuals who were previously infected with SARS-CoV-2, suggesting the sufficiency of a single dose to those individuals. However, these important data are limited to developed nations and lacking in resource-limited countries, like Ethiopia. Methods We compared receptor-binding domain (RBD)-specific IgG antibodies in 40 SARS-CoV-2 naïve participants and 25 participants previously infected with SARS-CoV-2, who received two doses of ChAdOx1 nCoV-19 vaccine. We measured the antibody response in post-vaccination blood samples from both groups of participants collected at four different post-vaccination time points: 8- and 12-weeks after each dose of the vaccine administration using an in-house developed ELISA. Results We observed a high level of anti-RBD IgG antibodies titers 8-weeks after a single dose administration (16/27; 59.3%) among naïve participants, albeit dropped significantly (p<0.05) two months later, suggesting the protective immunity elicited by the first dose ChAdOx1 nCoV-19 vaccine will likely last for a minimum of three months. However, as expected, a significant (p<0.001) increase in the level of anti-RBD IgG antibodies titers was observed after the second dose administration in all naïve participants. By contrast, the ChAdOx1 nCoV-19 vaccine-induced anti-RBD IgG antibody titers produced by the P.I participants at 8- to 12-weeks post-single dose vaccination were found to be similar to the antibody titers seen after a two-dose vaccination course among infection- naïve participants and showed no significant (p>0.05) increment following the second dose administration. Conclusion Taken together, our findings show that a single ChAdOx1 nCoV-19 dose in previously SARS-CoV-2 infected individuals elicits similar antibody responses to that of double dose vaccinated naïve individuals. Age and sex were not associated with the level of vaccine-elicited immune responses in both individuals with and without prior SARS-CoV-2 infection. Further studies are required to assess the need for a booster dose to extend the duration and amplitude of the specific protective immune response in Ethiopia settings, especially following the Omicron pandemic.
Conflict of interest statement
Competing Interests
The authors declare no competing interests.
Figures
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

