Kinetics and persistence of cellular and humoral immune responses to SARS-CoV-2 vaccine in healthcare workers with or without prior COVID-19
- PMID: 35043552
- PMCID: PMC8831971
- DOI: 10.1111/jcmm.17186
Kinetics and persistence of cellular and humoral immune responses to SARS-CoV-2 vaccine in healthcare workers with or without prior COVID-19
Abstract
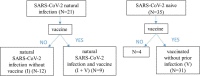
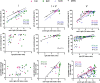
SARS-CoV-2 vaccines are highly efficient against severe forms of the disease, hospitalization and death. Nevertheless, insufficient protection against several circulating viral variants might suggest waning immunity and the need for an additional vaccine dose. We conducted a longitudinal study on the kinetics and persistence of immune responses in healthcare workers vaccinated with two doses of BNT162b2 mRNA vaccine with or without prior SARS-CoV-2 infection. No new infections were diagnosed during follow-up. At 6 months, post-vaccination or post-infection, despite a downward trend in the level of anti-S IgG antibodies, the neutralizing activity does not decrease significantly, remaining higher than 75% (85.14% for subjects with natural infection, 88.82% for vaccinated after prior infection and 78.37% for vaccinated only). In a live-virus neutralization assay, the highest neutralization titres were present at baseline and at 6 months follow-up in persons vaccinated after prior infection. Anti-S IgA levels showed a significant descending trend in vaccinated subjects (p < 0.05) after 14 weeks. Cellular immune responses are present even in vaccinated participants with declining antibody levels (index ratio 1.1-3) or low neutralizing activity (30%-40%) at 6 months, although with lower T-cell stimulation index (p = 0.046) and IFN-γ secretion (p = 0.0007) compared to those with preserved humoral responses.
Keywords: IFN-γ; SARS-CoV-2 infection; antibody levels; immune response post-infection; immune response post-vaccination; mRNA vaccine; neutralizing antibodies; spike-specific CD4+ T cell; spike-specific CD8+ T cell.
© 2022 The Authors. Journal of Cellular and Molecular Medicine published by Foundation for Cellular and Molecular Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Ritchie H, Mathieu E & Rodés‐Guirao L et al. Coronavirus pandemic (COVID‐19); 2020. https://ourworldindata.org/coronavirus. Accessed December 01, 2021.
-
- Kim JH, Marks F, Clemens JD. Looking beyond COVID‐19 vaccine phase 3 trials. Nat Med. 2021;27(2):205‐211. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

