6,5-Fused Ring, C2-Salvinorin Ester, Dual Kappa and Mu Opioid Receptor Agonists as Analgesics Devoid of Anxiogenic Effects
- PMID: 35043597
- PMCID: PMC9015904
- DOI: 10.1002/cmdc.202100684
6,5-Fused Ring, C2-Salvinorin Ester, Dual Kappa and Mu Opioid Receptor Agonists as Analgesics Devoid of Anxiogenic Effects
Abstract
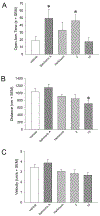
Current common analgesics are mediated through the mu or kappa opioid receptor agonism. Unfortunately, selective mu or kappa receptor agonists often cause harmful side effects. However, ligands exhibiting dual agonism to the opioid receptors, such as to mu and kappa, or to mu and delta, have been suggested to temper undesirable adverse effects while retaining analgesic activity. Herein we report an introduction of various 6,5-fused rings to C2 of the salvinorin scaffold via an ester linker. In vitro studies showed that many of these compounds have dual agonism on kappa and mu opioid receptors. In vivo studies on the lead dual kappa and mu opioid receptor agonist demonstrated supraspinal thermal analgesic activity while avoiding anxiogenic effects in male mice, thus providing further strong evidence in support of the therapeutic advantages of dual opioid receptor agonists over selective opioid receptor agonists.
© 2022 Wiley-VCH GmbH.
Conflict of interest statement
Conflict of Interest
The authors declare no conflict of interest.
Figures
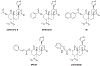
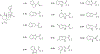



References
-
- Hooten WM, Mayo Clin. Proc 2016, 91, 955–970. - PubMed
-
- Vowles KE, McEntee ML, Julnes PS, Frohe T, Ney JP, van der Goes DN, Pain 2015, 156, 569–576. - PubMed
-
- Sałaga M, Polepally PR, Sobczak M, Grzywacz D, Kamysz W, Sibaev A, Storr M, Do Rego JC, Zjawiony JK, Fichna J, J. Pharmacol. Exp. Ther 2014, 350, 69–78. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

