Kernel Flow: a high channel count scalable time-domain functional near-infrared spectroscopy system
- PMID: 35043610
- PMCID: PMC8765296
- DOI: 10.1117/1.JBO.27.7.074710
Kernel Flow: a high channel count scalable time-domain functional near-infrared spectroscopy system
Abstract
Significance: Time-domain functional near-infrared spectroscopy (TD-fNIRS) has been considered as the gold standard of noninvasive optical brain imaging devices. However, due to the high cost, complexity, and large form factor, it has not been as widely adopted as continuous wave NIRS systems.
Aim: Kernel Flow is a TD-fNIRS system that has been designed to break through these limitations by maintaining the performance of a research grade TD-fNIRS system while integrating all of the components into a small modular device.
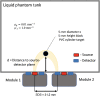
Approach: The Kernel Flow modules are built around miniaturized laser drivers, custom integrated circuits, and specialized detectors. The modules can be assembled into a system with dense channel coverage over the entire head.
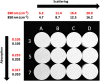
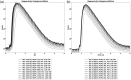
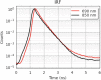
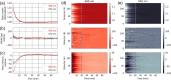
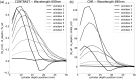
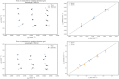
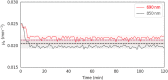
Results: We show performance similar to benchtop systems with our miniaturized device as characterized by standardized tissue and optical phantom protocols for TD-fNIRS and human neuroscience results.
Conclusions: The miniaturized design of the Kernel Flow system allows for broader applications of TD-fNIRS.
Keywords: functional near-infrared spectroscopy; optical brain imaging; optical properties; single-photon detectors; time-resolved spectroscopy; tissue optics.
Figures










References
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

