Development of a Novel Insulin Sensor for Clinical Decision-Making
- PMID: 35043720
- PMCID: PMC10347992
- DOI: 10.1177/19322968211071132
Development of a Novel Insulin Sensor for Clinical Decision-Making
Abstract
Background: Clinical decision support systems that incorporate information from frequent insulin measurements to enhance individualized diabetes management remain an unmet goal. The development of a disposable insulin strip for fast decentralized point-of-care detection replacing the current centralized lab-based methods used in clinical practice would be highly desirable to improve the establishment of individual insulin absorption patterns and algorithm modeling processes.
Methods: We carried out the development and optimization of a novel decentralized disposable insulin electrochemical sensor focusing on obtaining high analytical and operational performance toward achieving a true point-of-care insulin testing device for clinical on-site application.
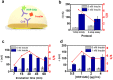
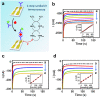
Results: Our novel insulin immunosensor demonstrated an attractive performance and efficient user-friendly operation by providing high sensitivity capability to detect endogenous and analog insulin with a limit of detection of 30.2 pM (4.3 µiU/mL), rapid time-to-result, stability toward remote site application, and scalable low-cost fabrication with an estimated cost-of-goods for disposable consumables of below $5, capable of near real-time insulin detection in a microliter (≤10 µL) sample droplet of undiluted serum within 30 minutes.
Conclusions: The results obtained in the optimization and characterization of our novel insulin sensor illustrate its suitability for its potential application in remote clinical environments for frequent insulin monitoring. Future work will test the insulin sensor in a clinical research setting to assess its efficacy in individuals with type 1 diabetes.
Keywords: immunosensor; insulin measurement; point-of-care; type 1 diabetes.
Conflict of interest statement
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: J.E.P. is currently an employee and shareholder of Tandem Diabetes Care, Inc. The work presented in the article was performed as part of his academic appointment at Sansum Diabetes Research Institute and is independent of his employment with Tandem Diabetes Care. E.D. reports receiving grants from Juvenile Diabetes Research Foundation, National Institutes of Health, and Helmsley Charitable Trust; personal fees from Roche and Eli Lilly; patents on artificial pancreas technology; and product support from Dexcom, Insulet, Tandem, and Roche. E.D. is currently an employee and shareholder of Eli Lilly and Company. The work presented in this article was performed as part of his academic appointment and is independent of his employment with Eli Lilly and Company. F.J.D. reports equity, licensed IP, and is a member of the Scientific Advisory Board of Mode AGC. L.M.L. reports grant support to her institution from National Institutes of Health, Juvenile Diabetes Research Foundation, Helmsley Charitable Trust, Eli Lilly and Company, Insulet, Dexcom, and Boehringer Ingelheim; she receives consulting fees unrelated to the current report from Johnson & Johnson, Sanofi, NovoNordisk, Roche, Dexcom, Insulet, Boehringer Ingelheim, ConvaTec, Medtronic, Lifescan, Laxmi, and Insulogic. M.-E.P. reports receiving grant support, provided to her institution, from National Institutes of Health, Helmsley Charitable Trust, Chan Zuckerberg Foundation, and Dexcom; patents related to hypoglycemia and pump therapy for hypoglycemia; and advisory board fees from Fractyl (unrelated to the current report). F.T. and H.T. are currently employees of ActioX LLC. The work presented in the article was performed as part of their academic appointment at University of California San Diego. All other authors report no conflict of interest.
Figures



References
-
- O’Connor P, Sperl-Hillen J.Current status and future directions for electronic point-of-care clinical decision support to improve diabetes management in primary care. Diabetes Technol Ther. 2019;21(2):26-34. - PubMed
-
- Shortliffe E, Sepúlveda M.Clinical decision support in the era of artificial intelligence. JAMA. 2018;320(21):2199-2200. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

