Novel VHH-Based Tracers with Variable Plasma Half-Lives for Imaging of CAIX-Expressing Hypoxic Tumor Cells
- PMID: 35044182
- PMCID: PMC9533306
- DOI: 10.1021/acs.molpharmaceut.1c00841
Novel VHH-Based Tracers with Variable Plasma Half-Lives for Imaging of CAIX-Expressing Hypoxic Tumor Cells
Abstract
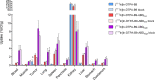
Hypoxic areas are present in the majority of solid tumors, and hypoxia is associated with resistance to therapies and poor outcomes. A transmembrane protein that is upregulated by tumor cells that have adapted to hypoxic conditions is carbonic anhydrase IX (CAIX). Therefore, noninvasive imaging of CAIX could be of prognostic value, and it could steer treatment strategies. The aim of this study was to compare variants of CAIX-binding VHH B9, with and without a C-terminal albumin-binding domain with varying affinity (ABDlow and ABDhigh), for SPECT imaging of CAIX expression. The binding affinity and internalization of the various B9-variants were analyzed using SK-RC-52 cells. Biodistribution studies were performed in mice with subcutaneous SCCNij153 human head and neck cancer xenografts. Tracer uptake was determined by ex vivo radioactivity counting and visualized by SPECT/CT imaging. Furthermore, autoradiography images of tumor sections were spatially correlated with CAIX immunohistochemistry. B9-variants demonstrated a similar moderate affinity for CAIX in vitro. Maximal tumor uptake and acceptable tumor-to-blood ratios were found in the SCCNij153 model at 4 h post injection for [111In]In-DTPA-B9 (0.51 ± 0.08%ID/g and 8.1 ± 0.85, respectively), 24 h post injection for [111In]In-DTPA-B9-ABDlow (2.39 ± 0.44%ID/g and 3.66 ± 0.81, respectively) and at 72 h post injection for [111In]In-DTPA-B9-ABDhigh (8.7 ± 1.34%ID/g and 2.43 ± 0.15, respectively). An excess of unlabeled monoclonal anti-CAIX antibody efficiently inhibited tumor uptake of [111In]In-DTPA-B9, while only a partial reduction of [111In]In-DTPA-B9-ABDlow and [111In]In-DTPA-B9-ABDhigh uptake was found. Immunohistochemistry and autoradiography images showed colocalization of all B9-variants with CAIX expression; however, [111In]In-DTPA-B9-ABDlow and [111In]In-DTPA-B9-ABDhigh also accumulated in non-CAIX expressing regions. Tumor uptake of [111In]In-DTPA-B9-ABDlow and [111In]In-DTPA-B9-ABDhigh, but not of [111In]In-DTPA-B9, could be visualized with SPECT/CT imaging. In conclusion, [111In]In-DTPA-B9 has a high affinity to CAIX and shows specific targeting to CAIX in head and neck cancer xenografts. The addition of ABD prolonged plasma half-life, increased tumor uptake, and enabled SPECT/CT imaging. This uptake was, however, partly CAIX- independent, precluding the ABD-tracers for use in hypoxia quantification in this tumor type.
Keywords: albumin-binding domain (ABD); carbonic anhydrase IX (CAIX); tumor hypoxia; variable domain of heavy chain only antibody (VHH).
Conflict of interest statement
The authors declare the following competing financial interest(s): Paul van Bergen en Henegouwen owns stocks of QVQ BV and LinXis BV.
Figures





References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

