Contribution of RNA-RNA Interactions Mediated by the Genome Packaging Signals for the Selective Genome Packaging of Influenza A Virus
- PMID: 35044211
- PMCID: PMC8941900
- DOI: 10.1128/JVI.01641-21
Contribution of RNA-RNA Interactions Mediated by the Genome Packaging Signals for the Selective Genome Packaging of Influenza A Virus
Abstract
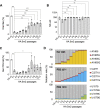
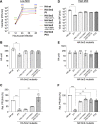
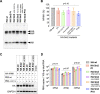
The influenza A virus genome is composed of eight single-stranded negative-sense viral RNA segments (vRNAs). The eight vRNAs are selectively packaged into each progeny virion. This process likely involves specific interactions between the vRNAs via segment-specific packaging signals located in both the 3'- and 5'-terminal regions of the respective vRNAs. To assess the importance of vRNA-vRNA interactions via packaging signals for selective genome packaging, we generated mutant viruses possessing silent mutations in the packaging signal region of the hemagglutinin (HA) vRNA. A mutant virus possessing silent mutations in nucleotides (nt) 1664 to 1676 resulted in defects in HA vRNA incorporation and showed a reduction in viral growth. After serial passage, the mutant virus acquired additional mutations in the 5'-terminal packaging signal regions of both the HA and polymerase basic 2 (PB2) vRNAs. These mutations contributed to the recovery of viral growth and HA vRNA packaging efficiency. In addition, an RNA-RNA interaction between the 5' ends of HA and PB2 vRNAs was confirmed in vitro, and this interaction was disrupted following the introduction of silent mutations in the HA vRNA. Thus, our results demonstrated that RNA-RNA interactions between the packaging signal regions of HA vRNA and PB2 vRNA are important for selective genome packaging. IMPORTANCE While numerous viral genomes comprise a single genome segment, the influenza A virus possesses eight segmented genomes. Influenza A virus can benefit from having a segmented genome because the segments can reassort with other strains of the influenza virus to create new genetically distinct strains. The influenza A virus efficiently incorporates one copy of each of its eight genomic segments per viral particle. However, the mechanism by which each segment is specifically selected is poorly understood. The genome segments contain RNA signals that facilitate the incorporation of segments into virus particles. These regions may facilitate specific interactions between the genome segments, creating an eight-segment complex, which can then be packaged into individual particles. In this study, we provide evidence that RNA signals contribute to specific interactions between two of the influenza virus genome segments.
Keywords: genome; genome packaging; influenza virus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
The genome-packaging signal of the influenza A virus genome comprises a genome incorporation signal and a genome-bundling signal.J Virol. 2013 Nov;87(21):11316-22. doi: 10.1128/JVI.01301-13. Epub 2013 Aug 7. J Virol. 2013. PMID: 23926345 Free PMC article.
-
In vitro vRNA-vRNA interactions in the H1N1 influenza A virus genome.Microbiol Immunol. 2020 Mar;64(3):202-209. doi: 10.1111/1348-0421.12766. Epub 2020 Jan 9. Microbiol Immunol. 2020. PMID: 31840833
-
Specific residues of the influenza A virus hemagglutinin viral RNA are important for efficient packaging into budding virions.J Virol. 2007 Sep;81(18):9727-36. doi: 10.1128/JVI.01144-07. Epub 2007 Jul 18. J Virol. 2007. PMID: 17634232 Free PMC article.
-
Selective Genome Packaging Mechanisms of Influenza A Viruses.Cold Spring Harb Perspect Med. 2021 Jul 1;11(7):a038497. doi: 10.1101/cshperspect.a038497. Cold Spring Harb Perspect Med. 2021. PMID: 32839251 Free PMC article. Review.
-
Packaging signal of influenza A virus.Virol J. 2021 Feb 17;18(1):36. doi: 10.1186/s12985-021-01504-4. Virol J. 2021. PMID: 33596956 Free PMC article. Review.
Cited by
-
Bipartite viral RNA genome heterodimerization influences genome packaging and virion thermostability.J Virol. 2024 Mar 19;98(3):e0182023. doi: 10.1128/jvi.01820-23. Epub 2024 Feb 8. J Virol. 2024. PMID: 38329331 Free PMC article.
-
Locations and structures of influenza A virus packaging-associated signals and other functional elements via an in silico pipeline for predicting constrained features in RNA viruses.PLoS Comput Biol. 2024 Apr 22;20(4):e1012009. doi: 10.1371/journal.pcbi.1012009. eCollection 2024 Apr. PLoS Comput Biol. 2024. PMID: 38648223 Free PMC article.
-
In vivo secondary structural analysis of Influenza A virus genomic RNA.Cell Mol Life Sci. 2023 May 2;80(5):136. doi: 10.1007/s00018-023-04764-1. Cell Mol Life Sci. 2023. PMID: 37131079 Free PMC article.
-
Sequential disruption of SPLASH-identified vRNA-vRNA interactions challenges their role in influenza A virus genome packaging.Nucleic Acids Res. 2023 Jul 7;51(12):6479-6494. doi: 10.1093/nar/gkad442. Nucleic Acids Res. 2023. PMID: 37224537 Free PMC article.
-
In vitro one-pot construction of influenza viral genomes for virus particle synthesis based on reverse genetics system.PLoS One. 2024 Nov 8;19(11):e0312776. doi: 10.1371/journal.pone.0312776. eCollection 2024. PLoS One. 2024. PMID: 39514509 Free PMC article.
References
-
- Fujii K, Fujii Y, Noda T, Muramoto Y, Watanabe T, Takada A, Goto H, Horimoto T, Kawaoka Y. 2005. Importance of both the coding and the segment-specific noncoding regions of the influenza A virus NS segment for its efficient incorporation into virions. J Virol 79:3766–3774. 10.1128/JVI.79.6.3766-3774.2005. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

