Mitochondrial Fusion, Fission, and Mitophagy in Cardiac Diseases: Challenges and Therapeutic Opportunities
- PMID: 35044229
- PMCID: PMC9125524
- DOI: 10.1089/ars.2021.0145
Mitochondrial Fusion, Fission, and Mitophagy in Cardiac Diseases: Challenges and Therapeutic Opportunities
Abstract
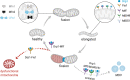
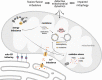
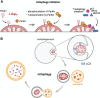
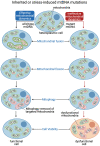
Significance: Mitochondria play a critical role in the physiology of the heart by controlling cardiac metabolism, function, and remodeling. Accumulation of fragmented and damaged mitochondria is a hallmark of cardiac diseases. Recent Advances: Disruption of quality control systems that maintain mitochondrial number, size, and shape through fission/fusion balance and mitophagy results in dysfunctional mitochondria, defective mitochondrial segregation, impaired cardiac bioenergetics, and excessive oxidative stress. Critical Issues: Pharmacological tools that improve the cardiac pool of healthy mitochondria through inhibition of excessive mitochondrial fission, boosting mitochondrial fusion, or increasing the clearance of damaged mitochondria have emerged as promising approaches to improve the prognosis of heart diseases. Future Directions: There is a reasonable amount of preclinical evidence supporting the effectiveness of molecules targeting mitochondrial fission and fusion to treat cardiac diseases. The current and future challenges are turning these lead molecules into treatments. Clinical studies focusing on acute (i.e., myocardial infarction) and chronic (i.e., heart failure) cardiac diseases are needed to validate the effectiveness of such strategies in improving mitochondrial morphology, metabolism, and cardiac function. Antioxid. Redox Signal. 36, 844-863.
Keywords: bioenergetic dysfunction; cardiovascular diseases; metabolism; mitochondrial dynamics; oxidative stress; therapy.
Conflict of interest statement
Patents on the design and application of mitochondrial fission peptide inhibitors have been filed. The authors claim that there is no conflict of interest related to this work.
Figures


References
-
- Abrahams JP, Leslie AGW, Lutter R, and Walker JE. Structure at 2.8 Â resolution of F1-ATPase from bovine heart mitochondria. Nature 370: 621–628, 1994. - PubMed
-
- Acín-Pérez R, Fernández-Silva P, Peleato ML, Pérez-Martos A, and Enriquez JA. Respiratory active mitochondrial supercomplexes. Mol Cell 32: P529–P539, 2008. - PubMed
-
- Acin-Perez R, Lechuga-Vieco AV, DelMarMuñoz M, Nieto-Arellano R, Torroja C, Sánchez-Cabo F, Jiménez C, González-Guerra A, Carrascoso I, Benincá C, Quiros PM, López-Otín C, Castellano JM, Ruíz-Cabello J, Jiménez-Borreguero LJ, and Enríquez JA. Ablation of the stress protease OMA1 protects against heart failure in mice. Sci Transl Med 10: eaan4935, 2018. - PubMed
-
- Amati-Bonneau P, Valentino ML, Reynier P, Gallardo ME, Bornstein B, Boissière A, Campos Y, Rivera H, De La Aleja JG, Carroccia R, Iommarini L, Labauge P, Figarella-Branger D, Marcorelles P, Furby A, Beauvais K, Letournel F, Liguori R, La Morgia C, Montagna P, Liguori M, Zanna C, Rugolo M, Cossarizza A, Wissinger B, Verny C, Schwarzenbacher R, Martín MÁ, Arenas J, Ayuso C, Garesse R, Lenaers G, Bonneau D, and Carelli V. OPA1 mutations induce mitochondrial DNA instability and optic atrophy “plus” phenotypes. Brain 131: 338–351, 2008. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

