Total Syntheses of Scabrolide A and Nominal Scabrolide B
- PMID: 35044751
- PMCID: PMC8815080
- DOI: 10.1021/jacs.1c12401
Total Syntheses of Scabrolide A and Nominal Scabrolide B
Abstract
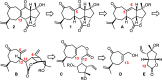
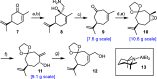
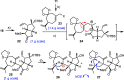
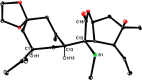
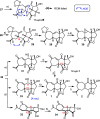
The marine natural product scabrolide A was obtained by isomerization of the vinylogous 1,4-diketone entity of nominal scabrolide B as the purported pivot point of the biosynthesis of these polycyclic norcembranoids. Despite the success of this maneuver, the latter compound itself turned out not to be identical with the natural product of that name. The key steps en route to the carbocyclic core of these targets were a [2,3]-sigmatropic rearrangement of an allylic sulfur ylide to forge the overcrowded C12-C13 bond, an RCM reaction to close the congested central six-membered ring, and a hydroxy-directed epoxidation/epoxide opening/isomerization sequence to set the "umpoled" 1,4-dicarbonyl motif and the correct angular configuration at C12.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- Palframan M. J.; Pattenden G. Biosynthetic Interrelationships within Polycyclic Cembranoids Isolated from Corals: Conjecture, Biomimetic Synthesis and Reality. Eur. J. Org. Chem. 2020, 2020 (16), 2330–2349. 10.1002/ejoc.201901438. - DOI
-
- The proposed transannular Michael addition could be emulated in the laboratory, whereas the retro-oxa-Micheal step has not been realized; see:Li Y.; Pattenden G. Tetrahedron 2011, 67, 10045–10052. 10.1016/j.tet.2011.09.040. - DOI
-
It is of note that the order of these biomimetic steps could also be reversed.
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

