CALGB 40603 (Alliance): Long-Term Outcomes and Genomic Correlates of Response and Survival After Neoadjuvant Chemotherapy With or Without Carboplatin and Bevacizumab in Triple-Negative Breast Cancer
- PMID: 35044810
- PMCID: PMC9015203
- DOI: 10.1200/JCO.21.01506
CALGB 40603 (Alliance): Long-Term Outcomes and Genomic Correlates of Response and Survival After Neoadjuvant Chemotherapy With or Without Carboplatin and Bevacizumab in Triple-Negative Breast Cancer
Abstract
Purpose: CALGB 40603 (NCT00861705), a 2 × 2 randomized phase II trial, demonstrated that adding carboplatin or bevacizumab to weekly paclitaxel (wP) followed by doxorubicin and cyclophosphamide significantly increased the pathologic complete response (pCR) rate in stage II-III triple-negative breast cancer. We now report long-term outcomes (LTOs) and correlative science end points.
Patients and methods: The Kaplan-Meier method was used to estimate LTOs in 443 patients who initiated study treatment. Log-rank tests and Cox proportional hazards models evaluated the impact of clinical characteristics, pathologic response, calculated residual cancer burden (RCB) in patients with residual disease (RD), treatment assignment, and dose delivery during wP on LTOs, including event-free survival (EFS). Genomic predictors of treatment response and outcomes were assessed on pretreatment tumor samples by mRNA sequencing.
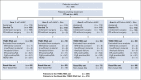
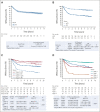
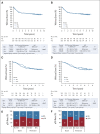
Results: Among baseline characteristics, only the clinical stage was associated with LTOs. At a median follow-up of 7.9 years, LTOs were not significantly improved with either carboplatin or bevacizumab, overall or in patients with basal-like subtype cancers by genomic analysis. Patients with pCR (n = 205, 46.3%) had significantly higher 5-year EFS (85.5% v 56.6%, log-rank P < .0001) and overall survival (87.9% v 63.4%, P < .0001) rates compared with patients with RD, even those with RCB class I. Among clinical and genomic features, evidence of immune activation, including tumor-infiltrating lymphocytes and low B-cell receptor evenness, was associated with pCR and improved EFS.
Conclusion: Despite higher pCR rates, neither carboplatin nor bevacizumab appeared to improve LTOs although the study was not powered to assess these secondary end points. pCR was associated with superior LTOs even when compared with minimal RD. Markers of immune activation in pretreatment tumor biopsies were independently associated with higher pCR rates and improved survival.
Conflict of interest statement
Figures

References
-
- Cortazar P, Zhang L, Untch M, et al. : Pathological complete response and long-term clinical benefit in breast cancer: The CTNeoBC pooled analysis. Lancet 384:164-172, 2014 - PubMed
-
- Sikov WM, Berry DA, Perou CM, et al. : Impact of the addition of carboplatin and/or bevacizumab to neoadjuvant once-per-week paclitaxel followed by dose-dense doxorubicin and cyclophosphamide on pathologic complete response rates in stage II to III triple-negative breast cancer: CALGB 40603 (Alliance). J Clin Oncol 33:13-21, 2015 - PMC - PubMed
-
- Symmans WF, Peintinger F, Hatzis C, et al. : Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J Clin Oncol 25:4414-4422, 2007 - PubMed
-
- Allison KH, Hammond MEH, Dowsett M, et al. : Estrogen and progesterone receptor testing in breast cancer: ASCO/CAP guideline update. J Clin Oncol 38:1346-1366, 2020 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- UG1 CA233290/CA/NCI NIH HHS/United States
- U10 CA180821/CA/NCI NIH HHS/United States
- UG1 CA233333/CA/NCI NIH HHS/United States
- P50 CA058223/CA/NCI NIH HHS/United States
- UG1 CA189858/CA/NCI NIH HHS/United States
- P30 CA008748/CA/NCI NIH HHS/United States
- UG1 CA233373/CA/NCI NIH HHS/United States
- UG1 CA233180/CA/NCI NIH HHS/United States
- U10 CA180882/CA/NCI NIH HHS/United States
- P30 CA033572/CA/NCI NIH HHS/United States
- UG1 CA233253/CA/NCI NIH HHS/United States
- U10 CA180888/CA/NCI NIH HHS/United States
- UG1 CA233339/CA/NCI NIH HHS/United States
- U24 CA196171/CA/NCI NIH HHS/United States
- P30 CA014236/CA/NCI NIH HHS/United States
- UG1 CA233327/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

