Recapitulating hepatitis E virus-host interactions and facilitating antiviral drug discovery in human liver-derived organoids
- PMID: 35044825
- PMCID: PMC8769558
- DOI: 10.1126/sciadv.abj5908
Recapitulating hepatitis E virus-host interactions and facilitating antiviral drug discovery in human liver-derived organoids
Abstract
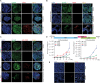
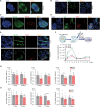
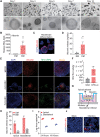
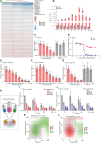
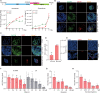
Hepatotropic viruses naturally have narrow host and tissue tropisms, challenging the development of robust experimental models. The advent of organoid technology provides a unique opportunity for moving the field forward. Here, we demonstrate that three-dimensional cultured organoids from fetal and adult human liver with cholangiocyte or hepatocyte phenotype support hepatitis E virus (HEV) replication. Inoculation with infectious HEV particles demonstrates that human liver–derived organoids support the full life cycle of HEV infection. By directing organoids toward polarized monolayers in a transwell system, we observed predominantly apical secretion of HEV particles. Genome-wide transcriptomic and tRNAome analyses revealed robust host responses triggered by viral replication. Drug screening in organoids identified brequinar and homoharringtonine as potent HEV inhibitors, which are also effective against the ribavirin resistance variant harboring G1634R mutation. Thus, successful recapitulation of HEV infection in liver-derived organoids shall facilitate the study of virus-host interactions and development of antiviral therapies.
Figures



References
-
- Wang Y., Metselaar H. J., Peppelenbosch M. P., Pan Q., Chronic hepatitis E in solid-organ transplantation: The key implications of immunosuppressants. Curr. Opin. Infect. Dis. 27, 303–308 (2014). - PubMed
-
- Hakim M. S., Wang W., Bramer W. M., Geng J., Huang F., de Man R. A., Peppelenbosch M. P., Pan Q., The global burden of hepatitis E outbreaks: A systematic review. Liver Int. 37, 19–31 (2017). - PubMed
-
- Ma X. X., Ji Y., Jin L., Baloch Z., Zhang D. R., Wang Y., Pan Q., Ma Z., Prevalence and clinical features of hepatitis E virus infection in pregnant women: A large cohort study in Inner Mongolia, China. Clin. Res. Hepatol. Gastroenterol. 45, 101536 (2020). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

