Class I histone deacetylases (HDAC1-3) are histone lysine delactylases
- PMID: 35044827
- PMCID: PMC8769552
- DOI: 10.1126/sciadv.abi6696
Class I histone deacetylases (HDAC1-3) are histone lysine delactylases
Abstract
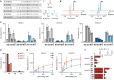
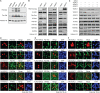
Lysine L-lactylation [K(L-la)] is a newly discovered histone mark stimulated under conditions of high glycolysis, such as the Warburg effect. K(L-la) is associated with functions that are different from the widely studied histone acetylation. While K(L-la) can be introduced by the acetyltransferase p300, histone delactylases enzymes remained unknown. Here, we report the systematic evaluation of zinc- and nicotinamide adenine dinucleotide–dependent histone deacetylases (HDACs) for their ability to cleave ε-N-L-lactyllysine marks. Our screens identified HDAC1–3 and SIRT1–3 as delactylases in vitro. HDAC1–3 show robust activity toward not only K(L-la) but also K(D-la) and diverse short-chain acyl modifications. We further confirmed the de-L-lactylase activity of HDACs 1 and 3 in cells. Together, these data suggest that histone lactylation is installed and removed by regulatory enzymes as opposed to spontaneous chemical reactivity. Our results therefore represent an important step toward full characterization of this pathway’s regulatory elements.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

