Quantitative, traceable determination of cell viability using absorbance microscopy
- PMID: 35045103
- PMCID: PMC8769294
- DOI: 10.1371/journal.pone.0262119
Quantitative, traceable determination of cell viability using absorbance microscopy
Abstract
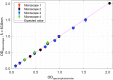
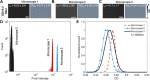
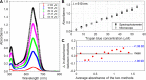
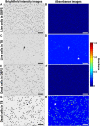
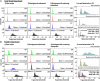
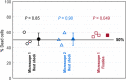
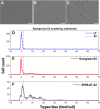
Cell viability, an essential measurement for cell therapy products, lacks traceability. One of the most common cell viability tests is trypan blue dye exclusion where blue-stained cells are counted via brightfield imaging. Typically, live and dead cells are classified based on their pixel intensities which may vary arbitrarily making it difficult to compare results. Herein, a traceable absorbance microscopy method to determine the intracellular uptake of trypan blue is demonstrated. The intensity pixels of the brightfield images are converted to absorbance images which are used to calculate moles of trypan blue per cell. Trypan blue cell viability measurements, where trypan blue content in each cell is quantified, enable traceable live-dead classifications. To implement the absorbance microscopy method, we developed an open-source AbsorbanceQ application that generates quantitative absorbance images. The validation of absorbance microscopy is demonstrated using neutral density filters. Results from four different microscopes demonstrate a mean absolute deviation of 3% from the expected optical density values. When assessing trypan blue-stained Jurkat cells, the difference in intracellular uptake of trypan blue in heat-shock-killed cells using two different microscopes is 3.8%. Cells killed with formaldehyde take up ~50% less trypan blue as compared to the heat-shock-killed cells, suggesting that the killing mechanism affects trypan blue uptake. In a test mixture of approximately 50% live and 50% dead cells, 53% of cells were identified as dead (±6% standard deviation). Finally, to mimic batches of low-viability cells that may be encountered during a cell manufacturing process, viability was assessed for cells that were 1) overgrown in the cell culture incubator for five days or 2) incubated in DPBS at room temperature for five days. Instead of making live-dead classifications using arbitrary intensity values, absorbance imaging yields traceable units of moles that can be compared, which is useful for assuring quality for biomanufacturing processes.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Bauer SR. Stem Cell-based Products in Medicine: FDA Regulatory Considerations. Handbook of Stem Cells. 2004:805–14. Epub 2007/09/28. doi: 10.1016/B978-012436643-5/50163-2 - DOI
-
- F2739 Standard Guide for Quantifying Cell Viability and Related Attributes within Biomaterial Scaffolds. West Conshohocken, PA: ASTM International; 2019.
-
- ISO 20391–1:2018 Biotechnology—Cell counting—Part 1: General guidance on cell counting methods. International Organization for Standardization.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

