A loop extrusion-independent mechanism contributes to condensin I-mediated chromosome shaping
- PMID: 35045152
- PMCID: PMC8932526
- DOI: 10.1083/jcb.202109016
A loop extrusion-independent mechanism contributes to condensin I-mediated chromosome shaping
Abstract
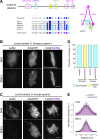
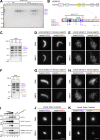
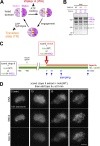
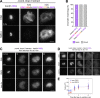
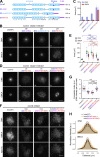
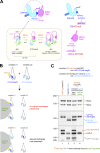
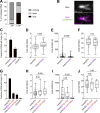
Condensin I is a five-subunit protein complex that is central to mitotic chromosome assembly in eukaryotic cells. Despite recent progress, its molecular mechanisms of action remain to be fully elucidated. By using Xenopus egg extracts as a functional assay, we find that condensin I complexes harboring mutations in its kleisin subunit CAP-H produce chromosomes with confined axes in the presence of topoisomerase IIα (topo IIα) and highly compact structures (termed "beans") with condensin-positive central cores in its absence. The bean phenotype depends on the SMC ATPase cycle and can be reversed by subsequent addition of topo IIα. The HEAT repeat subunit CAP-D2, but not CAP-G, is essential for the bean formation. Notably, loop extrusion activities of the mutant complexes cannot explain the chromosomal defects they exhibit in Xenopus egg extracts, implying that a loop extrusion-independent mechanism contributes to condensin I-mediated chromosome assembly and shaping. We provide evidence that condensin-condensin interactions underlie these processes.
© 2022 Kinoshita et al.
Figures





Similar articles
-
Functional interplay between condensin I and topoisomerase Iiα in single-molecule DNA compaction.Nat Commun. 2025 Aug 6;16(1):7239. doi: 10.1038/s41467-025-62600-5. Nat Commun. 2025. PMID: 40770194 Free PMC article.
-
Structural Basis of an Asymmetric Condensin ATPase Cycle.Mol Cell. 2019 Jun 20;74(6):1175-1188.e9. doi: 10.1016/j.molcel.2019.03.037. Mol Cell. 2019. PMID: 31226277 Free PMC article.
-
Contribution of hCAP-D2, a non-SMC subunit of condensin I, to chromosome and chromosomal protein dynamics during mitosis.Mol Cell Biol. 2005 Jan;25(2):740-50. doi: 10.1128/MCB.25.2.740-750.2005. Mol Cell Biol. 2005. PMID: 15632074 Free PMC article.
-
Taking cohesin and condensin in context.PLoS Genet. 2018 Jan 25;14(1):e1007118. doi: 10.1371/journal.pgen.1007118. eCollection 2018 Jan. PLoS Genet. 2018. PMID: 29370184 Free PMC article. Review.
-
The structure and function of SMC and kleisin complexes.Annu Rev Biochem. 2005;74:595-648. doi: 10.1146/annurev.biochem.74.082803.133219. Annu Rev Biochem. 2005. PMID: 15952899 Review.
Cited by
-
Cell cycle-specific loading of condensin I is regulated by the N-terminal tail of its kleisin subunit.Elife. 2022 Dec 13;11:e84694. doi: 10.7554/eLife.84694. Elife. 2022. PMID: 36511239 Free PMC article.
-
Polymer Modeling Reveals Interplay between Physical Properties of Chromosomal DNA and the Size and Distribution of Condensin-Based Chromatin Loops.Genes (Basel). 2023 Dec 9;14(12):2193. doi: 10.3390/genes14122193. Genes (Basel). 2023. PMID: 38137015 Free PMC article.
-
PfCAP-H is essential for assembly of condensin I complex and karyokinesis during asexual proliferation of Plasmodium falciparum.mBio. 2024 May 8;15(5):e0285023. doi: 10.1128/mbio.02850-23. Epub 2024 Apr 2. mBio. 2024. PMID: 38564676 Free PMC article.
-
Mitotic genome folding.J Cell Biol. 2025 Jul 7;224(7):e202504075. doi: 10.1083/jcb.202504075. Epub 2025 Jun 10. J Cell Biol. 2025. PMID: 40492990 Review.
-
Bridging-mediated compaction of mitotic chromosomes.Nucleus. 2025 Dec;16(1):2497765. doi: 10.1080/19491034.2025.2497765. Epub 2025 May 9. Nucleus. 2025. PMID: 40340634 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

