Lenvatinib plus Pembrolizumab for Advanced Endometrial Cancer
- PMID: 35045221
- PMCID: PMC11651366
- DOI: 10.1056/NEJMoa2108330
Lenvatinib plus Pembrolizumab for Advanced Endometrial Cancer
Abstract
Background: Standard therapy for advanced endometrial cancer after failure of platinum-based chemotherapy remains unclear.
Methods: In this phase 3 trial, we randomly assigned, in a 1:1 ratio, patients with advanced endometrial cancer who had previously received at least one platinum-based chemotherapy regimen to receive either lenvatinib (20 mg, administered orally once daily) plus pembrolizumab (200 mg, administered intravenously every 3 weeks) or chemotherapy of the treating physician's choice (doxorubicin at 60 mg per square meter of body-surface area, administered intravenously every 3 weeks, or paclitaxel at 80 mg per square meter, administered intravenously weekly [with a cycle of 3 weeks on and 1 week off]). The two primary end points were progression-free survival as assessed on blinded independent central review according to the Response Evaluation Criteria in Solid Tumors, version 1.1, and overall survival. The end points were evaluated in patients with mismatch repair-proficient (pMMR) disease and in all patients. Safety was also assessed.
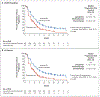
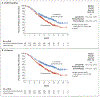
Results: A total of 827 patients (697 with pMMR disease and 130 with mismatch repair-deficient disease) were randomly assigned to receive lenvatinib plus pembrolizumab (411 patients) or chemotherapy (416 patients). The median progression-free survival was longer with lenvatinib plus pembrolizumab than with chemotherapy (pMMR population: 6.6 vs. 3.8 months; hazard ratio for progression or death, 0.60; 95% confidence interval [CI], 0.50 to 0.72; P<0.001; overall: 7.2 vs. 3.8 months; hazard ratio, 0.56; 95% CI, 0.47 to 0.66; P<0.001). The median overall survival was longer with lenvatinib plus pembrolizumab than with chemotherapy (pMMR population: 17.4 vs. 12.0 months; hazard ratio for death, 0.68; 95% CI, 0.56 to 0.84; P<0.001; overall: 18.3 vs. 11.4 months; hazard ratio, 0.62; 95% CI, 0.51 to 0.75; P<0.001). Adverse events of grade 3 or higher occurred in 88.9% of the patients who received lenvatinib plus pembrolizumab and in 72.7% of those who received chemotherapy.
Conclusions: Lenvatinib plus pembrolizumab led to significantly longer progression-free survival and overall survival than chemotherapy among patients with advanced endometrial cancer. (Funded by Eisai and Merck Sharp and Dohme [a subsidiary of Merck]; Study 309-KEYNOTE-775 ClinicalTrials.gov number, NCT03517449.).
Copyright © 2022 Massachusetts Medical Society.
Figures
Comment in
-
Survival benefit with second-line combination in endometrial cancer.Nat Rev Clin Oncol. 2022 Mar;19(3):149. doi: 10.1038/s41571-022-00604-6. Nat Rev Clin Oncol. 2022. PMID: 35087212 No abstract available.
-
Pembrolizumab plus lenvatinib for all advanced endometrial cancer? Maybe not.Int J Gynecol Cancer. 2022 Apr 4;32(4):579. doi: 10.1136/ijgc-2022-003419. Int J Gynecol Cancer. 2022. PMID: 35190462 No abstract available.
References
-
- Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res 2014;74:2913–21. - PubMed
-
- Lortet-Tieulent J, Ferlay J, Bray F, Jemal A. International patterns and trends in endometrial cancer incidence, 1978-2013. J Natl Cancer Inst 2018;110:354–61. - PubMed
-
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71:209–49. - PubMed
-
- Brooks RA, Fleming GF, Lastra RR, et al. Current recommendations and recent progress in endometrial cancer. CA Cancer J Clin 2019;69:258–79. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
