Immunogenicity and Reactogenicity of Vaccine Boosters after Ad26.COV2.S Priming
- PMID: 35045226
- PMCID: PMC8796791
- DOI: 10.1056/NEJMoa2116747
Immunogenicity and Reactogenicity of Vaccine Boosters after Ad26.COV2.S Priming
Abstract
Background: The Ad26.COV2.S vaccine, which was approved as a single-shot immunization regimen, has been shown to be effective against severe coronavirus disease 2019. However, this vaccine induces lower severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike protein (S)-specific antibody levels than those induced by messenger RNA (mRNA)-based vaccines. The immunogenicity and reactogenicity of a homologous or heterologous booster in persons who have received an Ad26.COV2.S priming dose are unclear.
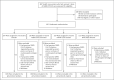
Methods: In this single-blind, multicenter, randomized, controlled trial involving health care workers who had received a priming dose of Ad26.COV2.S vaccine, we assessed immunogenicity and reactogenicity 28 days after a homologous or heterologous booster vaccination. The participants were assigned to receive no booster, an Ad26.COV2.S booster, an mRNA-1273 booster, or a BNT162b2 booster. The primary end point was the level of S-specific binding antibodies, and the secondary end points were the levels of neutralizing antibodies, S-specific T-cell responses, and reactogenicity. A post hoc analysis was performed to compare mRNA-1273 boosting with BNT162b2 boosting.
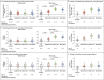
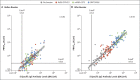
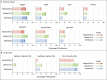
Results: Homologous or heterologous booster vaccination resulted in higher levels of S-specific binding antibodies, neutralizing antibodies, and T-cell responses than a single Ad26.COV2.S vaccination. The increase in binding antibodies was significantly larger with heterologous regimens that included mRNA-based vaccines than with the homologous booster. The mRNA-1273 booster was most immunogenic and was associated with higher reactogenicity than the BNT162b2 and Ad26.COV2.S boosters. Local and systemic reactions were generally mild to moderate in the first 2 days after booster administration.
Conclusions: The Ad26.COV2.S and mRNA boosters had an acceptable safety profile and were immunogenic in health care workers who had received a priming dose of Ad26.COV2.S vaccine. The strongest responses occurred after boosting with mRNA-based vaccines. Boosting with any available vaccine was better than not boosting. (Funded by the Netherlands Organization for Health Research and Development ZonMw; SWITCH ClinicalTrials.gov number, NCT04927936.).
Copyright © 2022 Massachusetts Medical Society.
Figures
References
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
