Microglia Activate Early Antiviral Responses upon Herpes Simplex Virus 1 Entry into the Brain to Counteract Development of Encephalitis-Like Disease in Mice
- PMID: 35045263
- PMCID: PMC8941881
- DOI: 10.1128/JVI.01311-21
Microglia Activate Early Antiviral Responses upon Herpes Simplex Virus 1 Entry into the Brain to Counteract Development of Encephalitis-Like Disease in Mice
Abstract
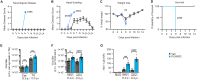
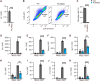
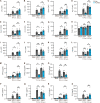
Spread of herpes simplex virus 1 (HSV1) from the periphery to the central nervous system (CNS) can lead to extensive infection and pathological inflammation in the brain, causing herpes simplex encephalitis (HSE). It has been shown that microglia, the CNS-resident macrophages, are involved in early sensing of HSV1 and induction of antiviral responses. In addition, infiltration of peripheral immune cells may contribute to the control of viral infection. In this study, we tested the effect of microglia depletion in a mouse model of HSE. Increased viral titers and increased disease severity were observed in microglia-depleted mice. The effect of microglia depletion was more pronounced in wild-type than in cGas-/- mice, revealing that this immune sensor contributes to the antiviral activity of microglia. Importantly, microglia depletion led to reduced production of type I interferon (IFN), proinflammatory cytokines, and chemokines at early time points after viral entry into the CNS. In line with this, in vitro experiments on murine primary CNS cells demonstrated microglial presence to be essential for IFN RNA induction, and control of HSV1 replication. However, the effect of microglia depletion on the expression of IFNs, and inflammatory cytokines was restricted to the early time point of HSV1 entry into the CNS. There was no major alteration of infiltration of CD45-positive cells in microglia-depleted mice. Collectively, our data demonstrate a key role for microglia in controlling HSV1 replication early after viral entry into the CNS and highlight the importance of a prompt antiviral innate response to reduce the risk of HSE development. IMPORTANCE One of the most devastating and acute neurological conditions is encephalitis, i.e., inflammation of brain tissue. Herpes simplex virus 1 (HSV1) is a highly prevalent pathogen in humans, and the most frequent cause of viral sporadic encephalitis called herpes simplex encephalitis (HSE). HSV1 can infect peripheral neurons and reach the central nervous system (CNS) of humans, where it can be detected by brain resident cells and infiltrating immune cells, leading to protective and damaging immune responses. In this study, we investigated the effects of microglia depletion, the main brain-resident immune cell type. For this purpose, we used a mouse model of HSE. We found that viral levels increased, and disease symptoms worsened in microglia-depleted mice. In addition, mice lacking a major sensor of viral DNA, cGAS, manifested a more pronounced disease than wild-type mice, highlighting the importance of this immune sensor in the activity of microglia. Microglia depletion led to reduced production of many known antiviral factors, most notably type I interferon (IFN). The importance of microglia in the early control of HSV1 spread and the generation of antiviral responses is further demonstrated by experiments on murine mixed glial cell cultures. Interestingly, mice with microglia depletion exhibited an unaltered activation of antiviral responses and recruitment of immune cells from the periphery at later time points of infection, but this did not prevent the development of the disease. Overall, the data highlight the importance of rapid activation of the host defense, with microglia playing a critical role in controlling HSV1 infection, which eventually prevents damage to neurons and brain tissue.
Keywords: DNA sensor; PLX5622; cGAS; central nervous system infections; herpes simplex virus; innate immunity; interferon; microglia.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



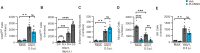

Similar articles
-
An Early Microglial Response Is Needed To Efficiently Control Herpes Simplex Virus Encephalitis.J Virol. 2020 Nov 9;94(23):e01428-20. doi: 10.1128/JVI.01428-20. Print 2020 Nov 9. J Virol. 2020. PMID: 32938766 Free PMC article.
-
Enhanced viral clearance and reduced leukocyte infiltration in experimental herpes encephalitis after intranasal infection of CXCR3-deficient mice.J Neurovirol. 2017 Jun;23(3):394-403. doi: 10.1007/s13365-016-0508-6. Epub 2017 Jan 23. J Neurovirol. 2017. PMID: 28116674
-
Sensing of HSV-1 by the cGAS-STING pathway in microglia orchestrates antiviral defence in the CNS.Nat Commun. 2016 Nov 10;7:13348. doi: 10.1038/ncomms13348. Nat Commun. 2016. PMID: 27830700 Free PMC article.
-
Microglial response to viral challenges: every silver lining comes with a cloud.Front Biosci (Landmark Ed). 2011 Jun 1;16(6):2187-205. doi: 10.2741/3847. Front Biosci (Landmark Ed). 2011. PMID: 21622170 Review.
-
Innate immune response during herpes simplex virus encephalitis and development of immunomodulatory strategies.Rev Med Virol. 2015 Sep;25(5):300-19. doi: 10.1002/rmv.1848. Epub 2015 Jul 23. Rev Med Virol. 2015. PMID: 26205506 Review.
Cited by
-
The role of STING signaling in central nervous system infection and neuroinflammatory disease.WIREs Mech Dis. 2023 May-Jun;15(3):e1597. doi: 10.1002/wsbm.1597. Epub 2023 Jan 12. WIREs Mech Dis. 2023. PMID: 36632700 Free PMC article. Review.
-
Temporally resolved single-cell RNA sequencing reveals protective and pathological responses during herpes simplex virus CNS infection.J Neuroinflammation. 2025 May 31;22(1):146. doi: 10.1186/s12974-025-03471-x. J Neuroinflammation. 2025. PMID: 40450318 Free PMC article.
-
CRISPR/Cas9 Eye Drop HSV-1 Treatment Reduces Brain Viral Load: A Novel Application to Prevent Neuronal Damage.Pathogens. 2024 Dec 10;13(12):1087. doi: 10.3390/pathogens13121087. Pathogens. 2024. PMID: 39770347 Free PMC article.
-
Orchestration of antiviral responses within the infected central nervous system.Cell Mol Immunol. 2024 Sep;21(9):943-958. doi: 10.1038/s41423-024-01181-7. Epub 2024 Jul 12. Cell Mol Immunol. 2024. PMID: 38997413 Free PMC article. Review.
-
HSV-1 immune escapes in microglia by down-regulating GM130 to inhibit TLR3-mediated innate immune responses.Virol J. 2024 Sep 16;21(1):219. doi: 10.1186/s12985-024-02492-x. Virol J. 2024. PMID: 39285274 Free PMC article.
References
-
- Murray PR, Rosenthal KS, Pfaller MA. 2016. Medical Microbiology 8th Edition Elsevier.
-
- Granerod J, Ambrose HE, Davies NWS, Clewley JP, Walsh AL, Morgan D, Cunningham R, Zuckerman M, Mutton KJ, Solomon T, Ward KN, Lunn MPT, Irani SR, Vincent A, Brown DWG, Crowcroft NS, UK Health Protection Agency (HPA) Aetiology of Encephalitis Study Group. 2010. Causes of encephalitis and differences in their clinical presentations in England: a multicentre, population-based prospective study. Lancet Infect Dis 10:835–844. 10.1016/S1473-3099(10)70222-X. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

