Safety and immunogenicity of a measles-vectored SARS-CoV-2 vaccine candidate, V591 / TMV-083, in healthy adults: results of a randomized, placebo-controlled Phase I study
- PMID: 35045362
- PMCID: PMC8761070
- DOI: 10.1016/j.ebiom.2021.103810
Safety and immunogenicity of a measles-vectored SARS-CoV-2 vaccine candidate, V591 / TMV-083, in healthy adults: results of a randomized, placebo-controlled Phase I study
Abstract
Background: V591 (TMV-083) is a live recombinant measles vector-based vaccine candidate expressing a pre-fusion stabilized SARS-CoV-2 spike protein.
Methods: We performed a randomized, placebo-controlled Phase I trial with an unblinded dose escalation and a double-blind treatment phase at 2 sites in France and Belgium to evaluate the safety and immunogenicity of V591. Ninety healthy SARS-CoV-2 sero-negative adults (18-55 years of age) were randomized into 3 cohorts, each comprising 24 vaccinees and 6 placebo recipients. Participants received two intramuscular injections of a low dose vaccine (1 × 105 median Tissue Culture Infectious Dose [TCID50]), one or two injections of a high dose vaccine (1 × 106 TCID50), or placebo with a 28 day interval. Safety was assessed by solicited and unsolicited adverse events. Immunogenicity was measured by SARS-CoV-2 spike protein-binding antibodies, neutralizing antibodies, spike-specific T cell responses, and anti-measles antibodies. ClinicalTrials.gov, NCT04497298.
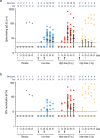
Findings: Between Aug 10 and Oct 13, 2020, 148 volunteers were screened of whom 90 were randomized. V591 showed a good safety profile at both dose levels. No serious adverse events were reported. At least one treatment-related adverse event was reported by 15 (20.8%) participants receiving V591 vs. 6 (33.3%) of participants receiving placebo. Eighty-one percent of participants receiving two injections of V591 developed spike-binding antibodies after the second injection. However, neutralizing antibodies were detectable on day 56 only in 17% of participants receiving the low dose and 61% receiving the high dose (2 injections). Spike-specific T cell responses were not detected. Pre-existing anti-measles immunity had a statistically significant impact on the immune response to V591, which was in contrast to previous results with the measles vector-based chikungunya vaccine.
Interpretation: While V591 was generally well tolerated, the immunogenicity was not sufficient to support further development.
Funding: Themis Bioscience GmbH, a subsidiary of Merck & Co. Inc., Kenilworth, NJ, USA; Coalition for Epidemic Preparedness Innovations (CEPI).
Keywords: COVID-19; SARS-CoV-2; measles vector; vaccine.
Copyright © 2022 Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ USA, The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests KR, RT, YT, AG, MM are employees of Themis Bioscience GmbH, a subsidiary of Merck & Co. Inc., Kenilworth, NJ, USA. KR, RT, MM possess stock options of Merck & Co. A patent application including the design of V591 has been filed by the Institut Pasteur and is part of a licensing agreement between the Institut Pasteur and Themis/MSD, NE and CG are inventors. CIC Cochin-Pasteur, SGS, INSERM, Bioaster received payment to conduct the study. All other authors declare no conflict of interest.
Figures


References
-
- World Health Organization. WHO COVID-19 Dashboard. https://covid19.who.int (accessed July 22, 2021).
-
- World Health Organization. Draft landscape of COVID-19 candidate vaccines. https://www.who.int/publications/m/item/draft-landscape-of-covid-19-cand... (accessed July 22, 2021).
-
- Haas EJ, Angulo FJ, McLaughlin JM, et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet. 2021;397(10287):1819–1829. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

