Randomized control trial on the efficacy of Limosilactobacillus reuteri ATCC PTA 4659 in reducing inflammatory markers in acute uncomplicated diverticulitis
- PMID: 35045564
- PMCID: PMC9936969
- DOI: 10.1097/MEG.0000000000002342
Randomized control trial on the efficacy of Limosilactobacillus reuteri ATCC PTA 4659 in reducing inflammatory markers in acute uncomplicated diverticulitis
Abstract
Introduction: Recent guidelines suggest treating acute uncomplicated diverticulitis (AUD) without antibiotics. We tested the efficacy of Limosilactobacillus reuteri ATCC PTA 4659 in AUD. Primary outcome was the reduction of abdominal pain and inflammatory markers [C-reactive protein (C-RP) and calprotectin]. Secondary outcome was the reduction of hours of hospitalization.
Patients and methods: A double-blind, randomized controlled trial was conducted in 119 patients with AUD. The probiotic group (61 patients) was treated with fluids, bowel rest and L. reuteri/b.i.d. for 10 days. The placebo group (58 patients) was treated with the same therapy and placebo/b.i.d. for 10 days. All patients completed a daily visual analogue scale (VAS) for abdominal pain.
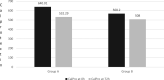
Results: Both groups showed a mean VAS score of 7 at enrolment and a reduction of 4 points after 3 days. C-RP value, after 72 h, decreased by 58.8% in the probiotic group and by only 40% in the placebo group (P < 0.05). Calprotectin levels, after 72 h, decreased by 17% in the probiotic group and by only 10.6% in the control group (P < 0.05). In the probiotic group, the hospitalization was done for 75.5 h compared to 83.5 in the placebo group.
Conclusions: The supplementation with L. reuteri 4659 together with bowel rest and fluids significantly reduced both blood and faecal inflammatory markers compared to the placebo group.
Copyright © 2022 Wolters Kluwer Health, Inc. All rights reserved.
Conflict of interest statement
There are no conflicts of interest.
Figures



References
-
- Simpson J. Recent advances in diverticular disease. Curr Gastroenterol Rep 2004; 6: 417–422. - PubMed
-
- Colonic diverticular disease. Nat Rev Dis Primers 2020; 6: 21. - PubMed
-
- Blair NP, Germann E. Surgical management of acute sigmoid diverticulitis. Am J Surg 2002; 183: 525–528. - PubMed
-
- Salzman H, Lillie D. Diverticular disease: diagnosis and treatment. Am Fam Physician 2005; 72: 1229–1234. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

